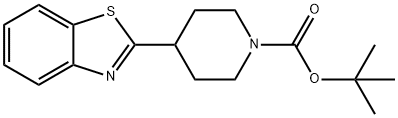
tert-Butyl 4-(1,3-benzothiazol-2-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(1,3-benzothiazol-2-yl)piperidine-1-carboxylate
- CAS Number:1373879-28-7
- Molecular formula:C17H22N2O2S
- Molecular Weight:318.43
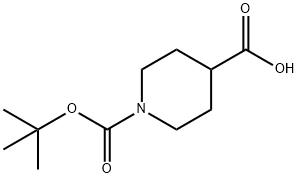
84358-13-4
391 suppliers
$5.00/5g
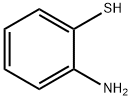
137-07-5
307 suppliers
$10.00/5g

1373879-28-7
24 suppliers
$27.00/100mg
Yield:1373879-28-7 97%
Reaction Conditions:
with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;N-ethyl-N,N-diisopropylamine in ethyl acetate at 100; for 0.166667 h;Microwave irradiation;Sealed tube;
Steps:
2.1 Synthesis of benzothiazoles
General procedure: A mixture of o-aminobenzenethiol (1 mmol), carboxylic acid (1 mmol), N,N-diisopropylethylamine (1.5 mmol) and propylphosphonic anhydride (1 mmol, 50% w/w in AcOEt) was irradiated for 10 min under microwave at 100 °C in a sealed tube. It was diluted with H2O, followed by alkalinization with saturated aqueous NaHCO3 solution. The precipitate was collected by filtration and washed thoroughly with H2O to afford the respective benzothiazole. If necessary, simple recrystallization was carried out in EtOH/H2O.
References:
Wen, Xiaoan;Bakali, Jamal El;Deprez-Poulain, Rebecca;Deprez, Benoit [Tetrahedron Letters,2012,vol. 53,# 19,p. 2440 - 2443] Location in patent:supporting information; experimental part
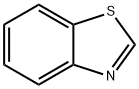
95-16-9
407 suppliers
$14.14/10gm:

84358-13-4
391 suppliers
$5.00/5g

1373879-28-7
24 suppliers
$27.00/100mg

2516-40-7
186 suppliers
$10.00/1g
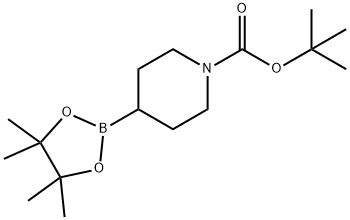
1048970-17-7
150 suppliers
$11.00/250mg

1373879-28-7
24 suppliers
$27.00/100mg

109384-19-2
484 suppliers
$5.00/1g

1373879-28-7
24 suppliers
$27.00/100mg