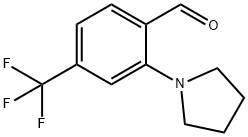
Benzaldehyde, 2-(1-pyrrolidinyl)-4-(trifluoromethyl)- synthesis
- Product Name:Benzaldehyde, 2-(1-pyrrolidinyl)-4-(trifluoromethyl)-
- CAS Number:1374603-97-0
- Molecular formula:C12H12F3NO
- Molecular Weight:243.23
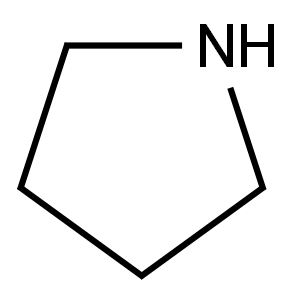
123-75-1
571 suppliers
$10.00/5g
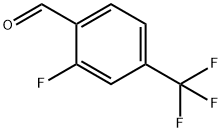
89763-93-9
171 suppliers
$10.00/1g

1374603-97-0
4 suppliers
inquiry
Yield:1374603-97-0 85%
Reaction Conditions:
with sodium hydrogencarbonate in acetonitrile at 20 - 76;
Steps:
8.1 Step 1:
Step 1:
Synthesis of 2-(pyrrolidin-1-yl)-4-(trifluoromethyl)benzaldehyde
A flask was charged with 2-fluoro-4-(trifluoromethyl)benzaldehyde (1.0 equiv.), ACN (5.4* Vol), and pyrrolidine (1.0 equiv.) The solution was cooled below 20° C. Solid sodium hydrogen carbonate (2.5 equiv.) was added, and the resulting slurry was heated to 76° C. until the reaction was complete by LC analysis.
The batch was cooled and diluted with water (?30* Vol) and EtOAc (?60* Vol), and the aqueous layer was separated and extracted with EtOAc (?30* Vol).
The organic layers were combined, washed with water (?30* Vol) and saturated aqueous sodium chloride (?30* Vol), and dried over anhydrous sodium sulfate.
The drying agent was removed by filtration, washed with EtOAc (?60* Vol), and the filtrate and rinse were concentrated under reduced pressure to an oil.
The oil was diluted with DCM (?13* Vol) and adsorbed onto silica gel (?8* Wt).
After removing solvent under reduced pressure, the dried silica gel was loaded onto a Biotage Ultra cartridge, and the product was eluted to provide 2-(pyrrolidin-1-yl)-4-(trifluoromethyl)benzaldehyde as a yellow oil in ?85% yield. 1H NMR (300 MHz, Chloroform-d) δ 10.14 (s, 1H), 7.79 (d, J=8.1 Hz, 1H), 7.05-6.99 (m, 2H), 3.42-3.37 (m, 4H), 2.05-2.01 (m, 4H); MS (ESI, m/z): 244.07 [M+H]+.
References:
US2018/134675,2018,A1 Location in patent:Paragraph 0315