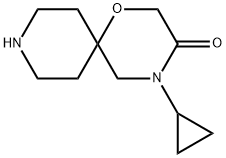
4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one synthesis
- Product Name:4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one
- CAS Number:1375142-88-3
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27
![1-Oxa-4,9-diazaspiro[5.5]undecan-3-one, 4-cyclopropyl-, hydrochloride (1:1)](/CAS/20211123/GIF/1375107-47-3.gif)
1375107-47-3
0 suppliers
inquiry
![4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one](/CAS/20180629/GIF/1375142-88-3.gif)
1375142-88-3
4 suppliers
inquiry
Yield:1375142-88-3 80%
Reaction Conditions:
with sodium hydroxide in dichloromethane;water; for 1 h;
Steps:
5.a a) 4-cyclopropyl-l-oxa-4,9-diazaspiro[5.5]undecan-3-one
A suspension of 4-cyclopropyl-l-oxa-4,9-diazaspiro[5.5]undecan-3-one hydrochloride (8.11 mmol) in dichloromethane (10 mL) was treated with IN aq NaOH (10.00 mmol). The biphasic mixture was stirred for 1 hour, at which point the cloudy organic layer had turned clear. The contents of the flask were further diluted with water (50 mL) and dichloromethane (50 mL) and the layers were separated. The aqueous layer was extracted with dichloromethane. The organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo to afford the title compound as a yellow oil (80%). MS(ES)+ m/e 211.2 [M+H]+.
References:
WO2013/28447,2013,A1 Location in patent:Page/Page column 56
![1-Piperidinecarboxylic acid, 4-[[(2-chloroacetyl)cyclopropylamino]methyl]-4-hydroxy-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1375107-45-1.gif)
1375107-45-1
0 suppliers
inquiry
![4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one](/CAS/20180629/GIF/1375142-88-3.gif)
1375142-88-3
4 suppliers
inquiry
![1-Oxa-4,9-diazaspiro[5.5]undecane-9-carboxylic acid, 4-cyclopropyl-3-oxo-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1375107-46-2.gif)
1375107-46-2
0 suppliers
inquiry
![4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one](/CAS/20180629/GIF/1375142-88-3.gif)
1375142-88-3
4 suppliers
inquiry
![1-OXA-6-AZASPIRO[2.5]OCTANE-6-CARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER](/CAS/GIF/147804-30-6.gif)
147804-30-6
210 suppliers
$6.00/250mg
![4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one](/CAS/20180629/GIF/1375142-88-3.gif)
1375142-88-3
4 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[(cyclopropylamino)methyl]-4-hydroxy-, 1,1-dimethylethyl ester](/CAS/GIF/1181759-11-4.gif)
1181759-11-4
3 suppliers
inquiry
![4-cyclopropyl-1-oxa-4,9-diazaspiro[5.5]undecan-3-one](/CAS/20180629/GIF/1375142-88-3.gif)
1375142-88-3
4 suppliers
inquiry