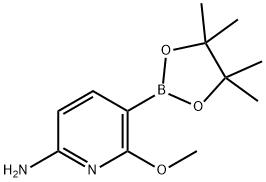
6-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amine synthesis
- Product Name:6-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amine
- CAS Number:1375708-04-5
- Molecular formula:C12H19BN2O3
- Molecular Weight:250.1

1211533-83-3
78 suppliers
$42.00/250mg

73183-34-3
564 suppliers
$6.00/5g

1375708-04-5
22 suppliers
$45.00/10mg
Yield:1375708-04-5 30%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 100; for 4 h;Inert atmosphere;
Steps:
121 Synthesis of 6-methoxy-5-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan-2-yl) pyridin-2-amine
To a stirred solution of 5-bromo-6-methoxypyridin-2-amine (3.5 g, 17.24 mmol) in 1, 4-dioxane (250 mL) under an argon atmosphere were added Bis (pinacolato) diboron (8.7 g, 34.48 mmol) and potassium acetate (5.08 g, 57.72 mmol) at room temperature and purged under an argon atmosphere for 30 min. Then Pd(dppf)Cl2 (3.8 g, 5.17 mmol) was added to the reaction mixture and stirred at 100 oC for 4 h. The reaction mixture was brought to room temperature and stirred for 1 h. After consumption of the starting material (monitored by TLC), the reaction was diluted with EtOAc (100 mL), filtered and the filtrate was concentrated in vacuo. The crude material was purified by column chromatography using 20% EtOAc:hexanes (neutralized with triethylamine) to afford 6-methoxy-5-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-amine (1.3 g, 30%) as a pale yellow solid. 1H- NMR (CDCl3, 400 MHz): δ 7.75 (d, 1H), 6.01 (d, 1H), 4.44 (br s, 2H), 3.88 (s, 3H), 1.31 (s, 12H); TLC: 30% EtOAc:hexanes (Rf: 0.3).
References:
WO2015/109109,2015,A1 Location in patent:Paragraph 0708
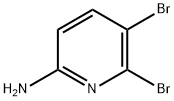
89284-11-7
99 suppliers
$17.00/100mg

1375708-04-5
22 suppliers
$45.00/10mg
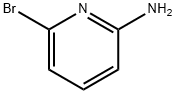
19798-81-3
434 suppliers
$5.00/1g

1375708-04-5
22 suppliers
$45.00/10mg