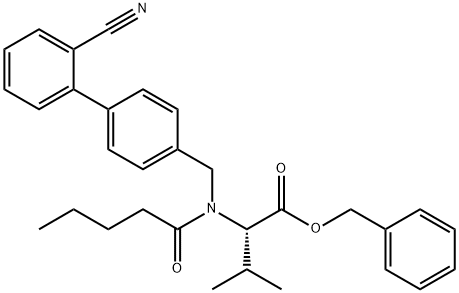
(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate synthesis
- Product Name:(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate
- CAS Number:137864-22-3
- Molecular formula:C31H34N2O3
- Molecular Weight:482.61
Yield:137864-22-3 98%
Reaction Conditions:
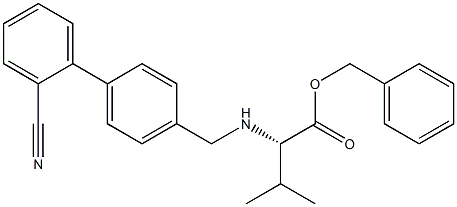
Stage #1: (S)-2-[(2'-cyanobiphenyl-4-ylmethyl)amino]-3-methylbutyric acid benzyl ester hydrochloridewith sodium hydrogencarbonate in water;toluene at 25; for 1 h;
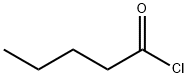
Stage #2: n-valeryl chloridewith N-ethyl-N,N-diisopropylamine in water;toluene at 15 - 25; for 0.5 - 1 h;
Stage #3: with hydrogenchloride in toluene at 0; pH=1 - 3; for 0.5 h;Product distribution / selectivity;
Steps:
4; 5 (S)-N-[(2'-cyanobiphenyl-4-yl)methyl]-N-valeroyl-(L)-valine benzyl ester (Formula III)
Example 4 (S)-N-[(2'-cyanobiphenyl-4-yl)methyl]-N-valeroyl-(L)-valine benzyl ester (Formula III) To sodium bicarbonate (65 g, 0.774 mol) in water (800 mL) and toluene (400 mL) was added (S)-N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-valine benzyl ester hydrochloride (210 g, 0.483 mol) at 25° C. The mixture was stirred for an hour; after which, the layers separated. The toluene layer was then washed with a brine solution and dried over sodium sulfate. To the dried toluene layer containing, (S)-N-[(2'-cyano biphenyl-4-yl)methyl]-(L)-valine benzyl ester, N,N-diisopropylethylamine (101.8 g, 0.786 mol) was added at 25° C. and cooled to 20° C. Valeroyl chloride (80 g, 0.664 mol) was then added over a period of about 30 minutes. After the completion of the reaction by thin layer chromatography (TLC) check, 50 mL water was added and further stirred for 30 minutes. The layers were separated; and IN hydrochloric acid solution was added to the organic layer at 0°C. until the pH reaches about 1-3. The acidified mixture was stirred for 30 minutes; and the layers separated. 10% sodium bicarbonate solution was added to the organic layer until the pH of reaction mass reaches about 7-8. The layers then separated; and the organic layer was washed with 200 mL water, and then with 200 mL brine solution. The organic layer was dried, solvent distilled out under reduced pressure to obtain 228 g (98% yield) of (S)-N-[(2'-cyanobiphenyl-4-yl)methyl]-N-valeroyl-(L)-valine benzyl ester as a brownish oil having a purity of 96% as measured by HPLC area percent. Example 5; (S)-N-(1-benzyloxycarbonyl-2-methyl-prop-1-yl)-N-pentanoyl-N-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amine (Formula IV) To a mixture of sodium bicarbonate (65 g, 0.774 mol), water (800 mL) and toluene (400 mL) was added (S)-N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-valine benzyl ester hydrochloride (210 g, 0.483 mol) at 25° C. and stirred for an hour. The layers were separated; and the toluene layer was dried over sodium sulfate. N,N-diisopropylethylanine (101.8 gm, 0.786 mol) was added to the dried toluene layer, containing (S)-N-[(2'-cyano biphenyl-4-yl)methyl]-(L)-valine benzyl ester, at 25° C. It was then cooled down to 15° C.; and valeryl chloride (80 g, 0.664 mol) was added over a period of about 60 minutes. Reaction progress monitored by TLC, on completion of reaction 100 mL water was added and further stirred for 30 minutes. The organic layer separated out and washed first with 1N hydrochloric acid solution until the pH was about 1-3, and then washed with 10% aqueous sodium bicarbonate solution until the pH of the washings reaches 7-8 respectively. The toluene layer was further washed with 200 mL water and then with 200 mL brine solution. The organic layer was subsequently dried using sodium sulfate. The dried organic layer was further used as such for tetrazole formation.
References:
US2006/281801,2006,A1 Location in patent:Page/Page column 5-6
114772-53-1
526 suppliers
$5.00/10g
![(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate](/CAS/20180703/GIF/137864-22-3.gif)
137864-22-3
5 suppliers
inquiry

21760-98-5
20 suppliers
$129.00/10g
![(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate](/CAS/20180703/GIF/137864-22-3.gif)
137864-22-3
5 suppliers
inquiry
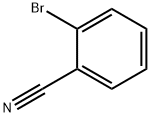
2042-37-7
352 suppliers
$7.00/10g
![(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate](/CAS/20180703/GIF/137864-22-3.gif)
137864-22-3
5 suppliers
inquiry
![L-Valine, N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/137864-24-5.gif)