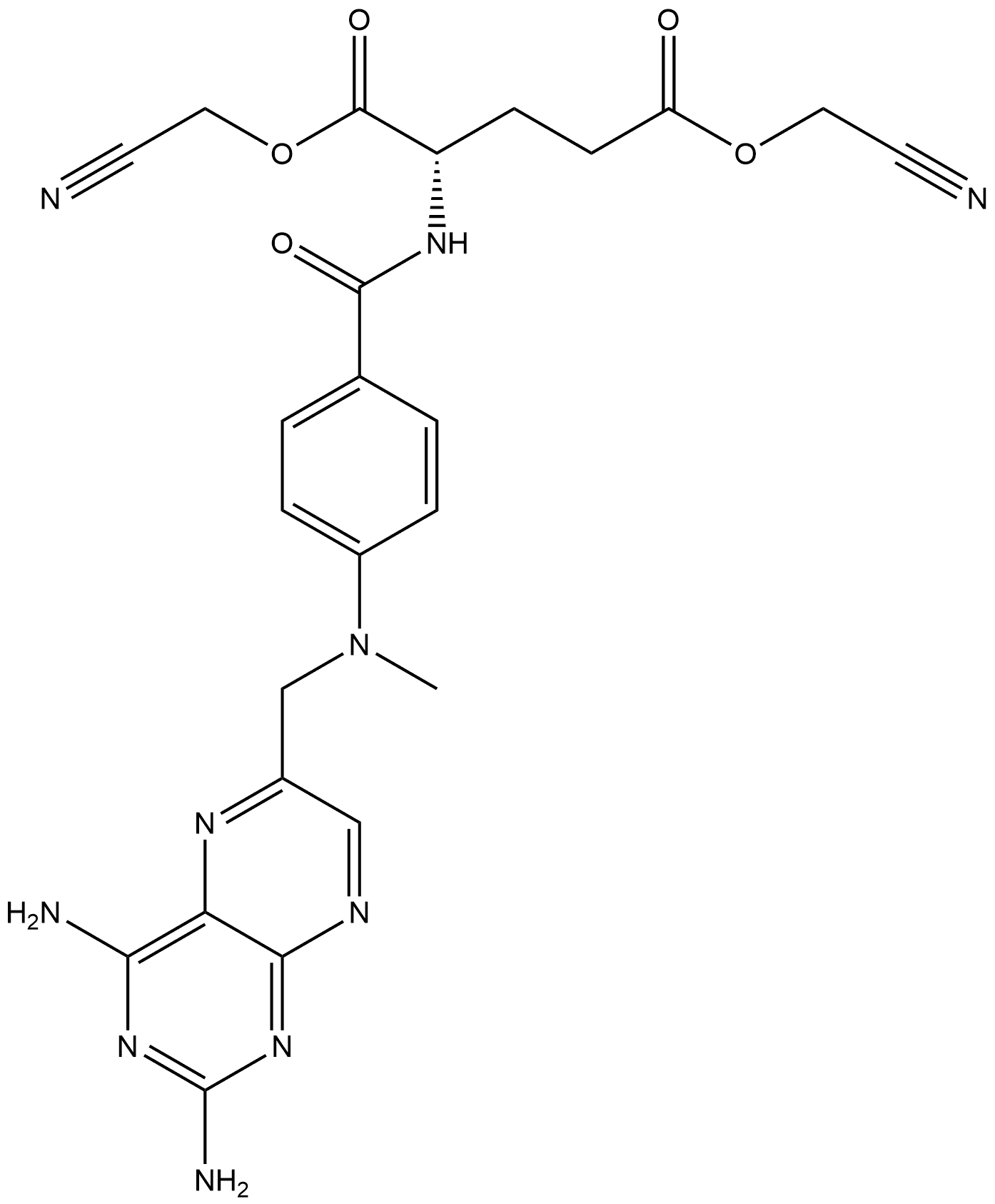
L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, 1,5-bis(cyanomethyl) ester synthesis
- Product Name:L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, 1,5-bis(cyanomethyl) ester
- CAS Number:1379609-57-0
- Molecular formula:C24H24N10O5
- Molecular Weight:532.51
![L-Glutamic acid, N-[4-(methylamino)benzoyl]-, 1,5-bis(cyanomethyl) ester](/CAS/20210305/GIF/1379609-56-9.gif)
1379609-56-9
0 suppliers
inquiry
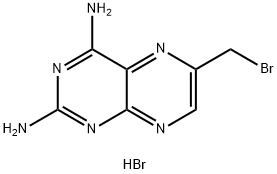
52853-40-4
156 suppliers
$21.00/250mg
![L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, 1,5-bis(cyanomethyl) ester](/CAS/20210305/GIF/1379609-57-0.gif)
1379609-57-0
0 suppliers
inquiry
Yield:1379609-57-0 87%
Reaction Conditions:
in water at 58 - 62; pH=2.4; for 1 h;
Steps:
3
A I L flask was equipped with magnetic stirrer, thermometer and condenser. The flask was charged with 143 ml of water and 10 g (0.029 mol) of 2,4- Diarnino-6-(bromomethyl)pteredine hydrobromide at room temperature. To this suspension was added 13 g (0.037 mol) of Dicyanomethyl N-[4- (methylamino) benzoyl] -L-glutamic acid at room temperature. The pH of the mixture was 2.40. The mixture was heated to 58-62 C and stirred there for 1 hour. The progress of the reaction was followed by TLC (EtOAc:MeOH, 4:1) and it was completed. The mixture was cooled to room temperature and the solid was isolated by filtration. The solid cake was washed with 15 ml of water and dried under vacuum at 50 °C for 5-6 hours to give 13.2 g (0.029 mol) of Methotrexate Dicyanomethyl ester as a yellow solid in 87% yield. According to 1H-NMR the product was very clean.1H-NMR (DMSO) 5 2 08(m, 2H), 2.55(t, 2H), 3.23(s, 3H), 4.46(m, 1H), 4.82(d, 2H), 4.94(s, 2H), 4.98 (s, 2H), 6.82(d, 2H), 7.35(m, 2H), 7.72(d, 2H), 8.30(s, 1H), 8.52(d, 2H), 8.64(s, 1H).
References:
WO2012/74496,2012,A1 Location in patent:Page/Page column 12