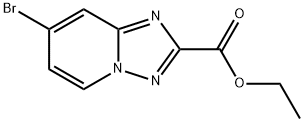
ETHYL 7-BROMO-[1,2,4]TRIAZOLO[1,5-A]PYRIDINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 7-BROMO-[1,2,4]TRIAZOLO[1,5-A]PYRIDINE-2-CARBOXYLATE
- CAS Number:1380331-36-1
- Molecular formula:C9H8BrN3O2
- Molecular Weight:270.08
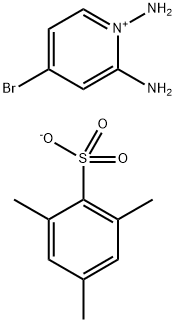
1202704-61-7
0 suppliers
inquiry
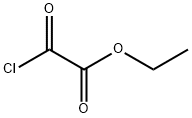
4755-77-5
331 suppliers
$9.00/25g
![ETHYL 7-BROMO-[1,2,4]TRIAZOLO[1,5-A]PYRIDINE-2-CARBOXYLATE](/CAS/20180601/GIF/1380331-36-1.gif)
1380331-36-1
21 suppliers
inquiry
Yield:1380331-36-1 60.5%
Reaction Conditions:
with pyridine at 100; for 18 h;
Steps:
33.a
a) ethyl 7-bromo-[1,2,4]triazolo[1,5-a]pyridine-2-carboxylate A mixture of 1,2-diamino-4-bromopyridinium2,4,6-trimethylbenzenesulfonate (4.18 g, 10.8 mmol) and ethyl 2-chloro-2-oxoacetate (2.4 ml, 21.5 mmol) in pyridine (25 ml) is heated for 18 hours to 100° C. The solvent is evaporated and the orange residue triturated with sat. aqueous sodium hydrogencarbonate solution for 2 hours. The solid is collected by filtration, washed several times with water and dried affording ethyl 7-bromo-[1,2,4]triazolo[1,5-a]pyridine-2-carboxylate (1.759 g, 60.5%) as a light pink solid. mp.: 158-160° C. MS: m/z=270.2 (M+H+).
References:
US2012/142665,2012,A1 Location in patent:Page/Page column 30
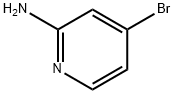
84249-14-9
362 suppliers
$6.00/1g
![ETHYL 7-BROMO-[1,2,4]TRIAZOLO[1,5-A]PYRIDINE-2-CARBOXYLATE](/CAS/20180601/GIF/1380331-36-1.gif)
1380331-36-1
21 suppliers
inquiry