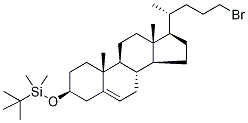
(3β)-24-BroMo-3-[(tert-butyl)diMethylsilyloxy]-chol-5-ene synthesis
- Product Name:(3β)-24-BroMo-3-[(tert-butyl)diMethylsilyloxy]-chol-5-ene
- CAS Number:1384736-07-5
- Molecular formula:C30H53BrOSi
- Molecular Weight:537.7307

84529-86-2
4 suppliers
inquiry
![(3β)-24-BroMo-3-[(tert-butyl)diMethylsilyloxy]-chol-5-ene](/CAS/GIF/1384736-07-5.gif)
1384736-07-5
6 suppliers
$140.00/5mg
Yield:1384736-07-5 95%
Reaction Conditions:
with 1H-imidazole;carbon tetrabromide;triphenylphosphine in diethyl ether;acetonitrile at 0 - 20;Inert atmosphere;
Steps:
4.2.19. (20R)-3β-(tert-Butyldimethylsilyloxy)-24-bromochol-5-ene (33)
CBr4 (99%, 198 mg, 0.59 mmol) was added portion-wise to a solution of alcohol 32 (140 mg, 0.29 mmol), PPh3 (99%, 168 mg, 0.63 mmol) and imidazole (102 mg, 1.50 mmol) in a mixture of anhydrous Et2O (3 mL) and CH3CN (1 mL) stirring under a N2 atmosphere at 0°C. The reaction mixture was allowed to warm to room temperature and stirred for 2.5 h, and then was re-cooled to 0 °C. MeOH (0.2 mL) was added to quench the reaction and the solvent was evaporated. The residue was purified by flash column chromatography (silica gel, 100% petroleum spirit 40-60 to 2% Et2O in petroleum spirit 40-60) to afford bromide 33 (151 mg, 0.28 mmol, 95%) as a white solid. Mp 108-110 °C. -10.0 (c 0.16, CHCl3). 1H NMR (400 MHz, CDCl3): δ 0.03 (s, 6H, ROSi(CH3)2C(CH3)3), 0.66 (s, 3H, H3-18), 0.74-2.05 (m, 38H, incl. 0.88 [s, 9H, ROSi(CH3)2C(CH3)3], 0.91 [d, 3H, J = 6.6 Hz, H3-21]), 0.98 [s, 3H, H3-19]), 2.14 (m, 1H), 2.24 (m, 1H), 3.35 (m, 2H, H2-24), 3.46 (m, 1H, H-3), 5.29 (m, 1H, H-6). 13C NMR (100 MHz, CDCl3): δ -4.6 (2C, ROSi(CH3)2C(CH3)3), 11.8 (C-18), 18.2 (ROSi(CH3)2C(CH3)3), 18.7 (C-21), 19.4 (C-19), 21.0, 24.2, 25.9 (3C, ROSi(CH3)2C(CH3)3), 28.2, 29.6, 31.89 (C-20), 31.90, 32.1, 34.45, 34.50, 35.2, 36.6 (C-10), 37.4, 39.8, 42.3 (C-13), 42.8, 50.2, 55.8, 56.8, 72.6 (C-3), 121.1 (C-6), 141.5 (C-5). GC-MS (EI): m/z (%) 536/538 (0.2/0.1, M+), 521/523 (1/1, M+ -CH3), 479/481 (33/38, M+ -tBu), 405/407 (7/3, M+ -OTBS), 401 (7), 325 (8), 283 (3), 255 (4), 227 (3), 213 (5), 199 (4), 161 (8), 159 (20), 151 (3), 149 (6), 145 (14), 131 (9), 123 (5), 121 (10), 115 (5), 107/109 (15/14), 95 (30), 93 (18), 81 (23), 79 (13), 75 (100), 69 (28), 57 (10), 55 (26), 41 (22). Anal. Calcd for C30H53BrOSi: C, 67.01; H, 9.93. Found: C, 66.61; H, 9.68. HRMS (ESI): Calcd for C30H53BrNaOSi ([M+Na]+): 559.2947/561.2926. Obsd: 559.2943/561.2940.
References:
Johnston, Jonathan B.;Singh, Arti A.;Clary, Anaelle A.;Chen, Chiung-Kuan;Hayes, Patricia Y.;Chow, Sharon;De Voss, James J.;Ortiz De Montellano, Paul R. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 13,p. 4064 - 4081] Location in patent:experimental part