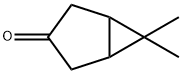
(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149) synthesis
- Product Name:(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149)
- CAS Number:13855-29-3
- Molecular formula:C8H12O
- Molecular Weight:124.18
![(1R,3S,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-Ol](/CAS/20180703/GIF/16613-81-3.gif)
16613-81-3
11 suppliers
$90.00/2mg
![(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149)](/CAS/20180808/GIF/13855-29-3.gif)
13855-29-3
8 suppliers
inquiry
Yield:13855-29-3 80%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane;Dess-Martin Oxidation;Reagent/catalyst;
Steps:
6. 6,6-Dimethylbicyclo[3.1.0]hexan-3-one (6):
[CAUTION: Literature1Error Bookmark not defined. has shown that Dess-Martin periodinane and IBXshould be treated as heat- and shock-sensitive compounds. Safety analysis shows exothermswhen heated (>130°C). All operations should be conducted with the appropriate safety equipment and procedures.] To a clean and dry 45 L reactor was charged dichloromethane(11,000 mL, 22V) and 5 (500 g, 3,96 mol). The resulting solution was cooled to 0 °C and theresulting solution was added Dess-Martin periodinane (2,017 g, 4.7 mol) in portions overapproximately 1 h while maintaining the internal temperature ?5°C. The reaction masstemperature was then warmed to 20 °C and stirred for 3h at 20-25 °C and the progress of thereaction was monitored by GC until 5 was not detected. The reaction was diluted withdichloromethane (10,000 mL, 20V) and filtered through a pad of celite and rinsed withdichloromethane (2,500 mL, 5V). The combined filtrate was washed twice with sodiumbicarbonate solution (5,000 mL, 10V) (Note: Slowly added NaHCO3 due to frothing), twice with10% w/v Na2S2O3 solution (5,000 mL, 10V) and brine (5,000 mL, 10V). Then the organic layerwas dried over Na2SO4, filtered and then concentrated under reduced pressure at 30-35°C toafford 490 g of 6 as pale yellow oil. GC purity: 96%. Assay by Quantitative NMR = 78% w/w. A267 g portion of the batch was then distilled at 1.5 mbar as temperature was slowly increasedfrom 20 °C to 80 °C (Condenser set point 5-10 °C, receiver set point -20 °C; Equipment: Shortpath distillation head with a combined cold-coil and Liebig condenser and affixed with a threeportdistribution adapter) and collected three fractions for a total of 212.5 g (80% yield ondistillation) of 6 as a clear colorless liquid.
References:
Mennen, Steven M.;Arunachalampillai, Athimoolam;Choquette, Deborah M.;Muppalla, Harikrishna;Sheena, Krishna [Synlett,2017,vol. 28,# 17,art. no. ST-2017-R0291-L,p. 2330 - 2334] Location in patent:supporting information
![(1R,3R,5S)-6,6-Dimethylbicyclo[3.1.0]hexan-3-ol](/CAS/20210305/GIF/16609-64-6.gif)
16609-64-6
1 suppliers
inquiry
![(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149)](/CAS/20180808/GIF/13855-29-3.gif)
13855-29-3
8 suppliers
inquiry
959963-31-6
1 suppliers
inquiry
![(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149)](/CAS/20180808/GIF/13855-29-3.gif)
13855-29-3
8 suppliers
inquiry
870-63-3
293 suppliers
$27.00/5g
![(1R,5S)-6,6-Dimethylbicyclo[3.1.0]Hexan-3-One(WX625149)](/CAS/20180808/GIF/13855-29-3.gif)
13855-29-3
8 suppliers
inquiry
![2-[2-[(E)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile](/CAS/20180601/GIF/96042-30-7.gif)