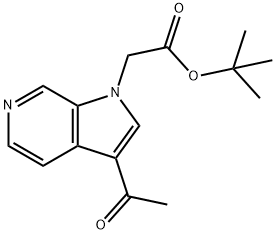
tert-Butyl 2-(3-acetyl-1H-pyrrolo[2,3-c]pyridin-1-yl)acetate synthesis
- Product Name:tert-Butyl 2-(3-acetyl-1H-pyrrolo[2,3-c]pyridin-1-yl)acetate
- CAS Number:1386457-04-0
- Molecular formula:C15H18N2O3
- Molecular Weight:274.32
5292-43-3
442 suppliers
$10.00/5g
![Ethanone, 1-(1H-pyrrolo[2,3-c]pyridin-3-yl)- (9CI)](/CAS/GIF/67058-71-3.gif)
67058-71-3
41 suppliers
$44.00/100mg
![tert-Butyl 2-(3-acetyl-1H-pyrrolo[2,3-c]pyridin-1-yl)acetate](/CAS/20180702/GIF/1386457-04-0.gif)
1386457-04-0
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-(1H-pyrrolo[2,3-c]pyridin-3-yl)ethanonewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;Inert atmosphere;
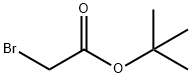
Stage #2: bromoacetic acid tert-butyl ester in N,N-dimethyl-formamide;mineral oil at 20; for 1.5 h;
Steps:
A
To a suspension of NaH (60 % in mineral oil, 90 mg, 2.25 mmol) in DMF (4.2 mL) cooled at 0°C was added a solution of 1-(1 H-pyrrolo[2,3-c]pyridin-3-yl)-ethanone (prepared according to J. Org. Chem. 2002, 67, 6226) (300 mg, 1.87 mmol) in DMF (4.2 mL) and the resulting suspension was stirred at 0°C under nitrogen atmosphere for 30 min before slow addition of tert-butyl bromoacetate (277 μ, 1.87 mmol). The reaction mixture was allowed to reach RT and further stirred at RT for 1.5 h. After completion of the reaction, the mixture was purified first by catch-release on SiliaPrep Tosic Acid-(2x10 g) (Varian) (eluent: MeOH (50 mL) by 2 M ammonia in MeOH (50 mL)) to give a mixture of regioisomers: (3-acetyl-pyrrolo[2,3-c]pyridin- 1-yl)-acetic acid tert-butyl ester and (3-acetyl-pyrrolo[2,3-c]pyridin-6-yl)-acetic acid tert-butyl ester followed by flash column chromatography on silica gel (CH2CI2 to CH2CI2/MeOH 85-15) to give (3-acetyl-pyrrolo[2,3-c]pyridin-1-yl)-acetic acid tert-butyl ester as a yellow powder. TLC, Rf (CH2CI2/ MeOH 9-1) = 0.50; MS (UPLC/MS): 275.3 [M+H]+, 319.2 [M+HCOO]-; tR (HPLC conditions f): 1.28 min; 1 H-NMR (400 MHz, DMSO-d6): δ (ppm): 8.88 (s, 1 H), 8.50 (s, 1 H), 8.33 (d, 1 H), 8.06 (d, 1 H), 5.27 (s, 2H), 2.48 (s,3H), 1.45 (s, 9H).
References:
WO2012/93101,2012,A1 Location in patent:Page/Page column 134-135