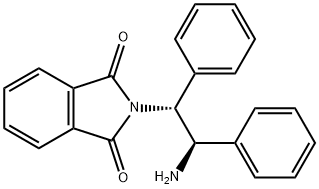
1H-Isoindole-1,3(2H)-dione, 2-[(1R,2R)-2-amino-1,2-diphenylethyl]- synthesis
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-[(1R,2R)-2-amino-1,2-diphenylethyl]-
- CAS Number:1387581-28-3
- Molecular formula:C22H18N2O2
- Molecular Weight:342.39
Yield:1387581-28-3 72%
Reaction Conditions:
with potassium carbonate;toluene-4-sulfonic acid in toluene; for 12 h;Reflux;Inert atmosphere;
Steps:
1 4.2.1 2-[(1R,2R)-2-Amino-1,2-diphenylethyl]-2,3-dihydro-1H-isoindole-1,3-dione (6) _
A solution of p-TsOH·H2O (5.70 g, 30 mmol) in toluene (30 mL) was dehydrated by azeotropic distillation (oil bath 120 °C). After being cooled to room temperature, (1R,2R)-1,2-diphenylethane-1,2-diamine (6.36 g, 30 mmol) was added to the solution, followed by phthalic anhydride (4.44 g, 30 mmol). The mixture was stirred at reflux for 12 h, then cooled to room temperature. The solid crude product was collected by filtration, washed with toluene-hexane and air-dried. The solid crude product was then added to a solution of saturated K2CO3 solution (150 mL) and CH2Cl2 (150 mL), and stirred overnight. After that, the organic solution was separated and extracted with CH2Cl2 (150 mL) to give combined organic layers, which were washed with brine, dried over MgSO4, and concentrated in vacuo to give 7.39 g (72%) of 6 as a white solid.
References:
Huang, Huayin;Zong, Hua;Shen, Bin;Yue, Huifeng;Bian, Guangling;Song, Ling [Tetrahedron,2014,vol. 70,# 6,p. 1289 - 1297]
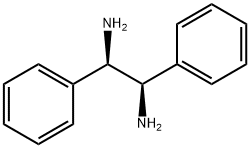
35132-20-8
363 suppliers
$7.00/1g
![1H-Isoindole-1,3(2H)-dione, 2-[(1R,2R)-2-amino-1,2-diphenylethyl]-](/CAS/20210305/GIF/1387581-28-3.gif)
1387581-28-3
7 suppliers
inquiry
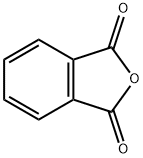
85-44-9
783 suppliers
$9.00/5g
![1H-Isoindole-1,3(2H)-dione, 2-[(1R,2R)-2-amino-1,2-diphenylethyl]-](/CAS/20210305/GIF/1387581-28-3.gif)
1387581-28-3
7 suppliers
inquiry