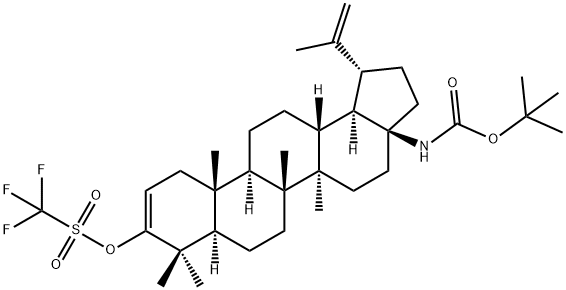
(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate synthesis
- Product Name:(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate
- CAS Number:1392315-38-6
- Molecular formula:C35H54F3NO5S
- Molecular Weight:657.87
Yield:1392315-38-6 72.3%
Reaction Conditions:
with 1,1,1,3,3,3-hexamethyldisilazane potassium in tetrahydrofuran at -78 - 20; for 2 h;Inert atmosphere;Concentration;Temperature;Time;
Steps:
2.6; A1.6 Step 6 Preparation of (1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate
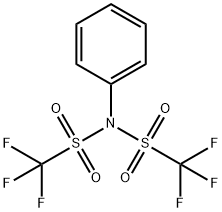
General procedure: Step 6 Preparation of (1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate A flask containing a solution of tert-butyl ((1R,3 aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-5a,5b,8,8,11a-pentamethyl-9-oxo-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysen-3a-yl)carbamate (1.2 g, 2.282 mmol) and 1,1,1-trifluoro-N-phenyl-N-(trifluoromethyl)sulfonyl methanesulfonamide (1.019 g, 2.85 mmol) in THF (20 mL) was cooled to -78° C. To the solution was added KHMDS (0.91 M in THF) (5.52 mL, 5.02 mmol). The mixture was stirred at -78° C. for 1 h then warmed to rt and stirred for 1 h. The reaction was then quenched with saturated 175 aqueous ammonium chloride (30 mL) and extracted with ethyl acetate (3×30 mL). The combined organic layers were dried over magnesium sulfate. The drying agent was removed by filtration and the filtrate was concentrated under reduced pressure. The crude material was purified using a 0-12% 126 ethyl acetate in hexanes gradient and a Thomson 80 g silica gel column. The fractions containing the expected product were combined and concentrated under reduced pressure to give (1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate (0.9 g, 1.368 mmol, 59.9% yield) as a white foam. 1H NMR (500 MHz, chloroform-d) δ=5.57 (dd, J=6.7, 1.8 Hz, 1H), 4.73 (s, 1H), 4.62 (s, 1H), 4.32 (br. s., 1H), 2.64-2.31 (m, 3H), 2.16 (dd, J=17.0, 6.8 Hz, 1H), 2.04-1.94 (m, 1H), 1.70 (s, 3H), 1.45 (s, 9H), 1.13 (s, 3H), 1.06 (s, 3H), 1.03 (s, 3H), 0.97 (s, 3H), 0.93 (s, 3H), 1.82-0.86 (m, 18H)
References:
US2013/210787,2013,A1 Location in patent:Paragraph 0170; 0181; 0182; 0294; 0300

472-15-1
414 suppliers
$5.00/25mg
![(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate](/CAS/20200611/GIF/1392315-38-6.gif)
1392315-38-6
2 suppliers
inquiry
![(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a-isocyanato-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysen-9-ol](/CAS/20180703/GIF/672958-04-2.gif)
672958-04-2
1 suppliers
inquiry
![(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate](/CAS/20200611/GIF/1392315-38-6.gif)
1392315-38-6
2 suppliers
inquiry

473-98-3
392 suppliers
$16.35/10mg
![(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate](/CAS/20200611/GIF/1392315-38-6.gif)
1392315-38-6
2 suppliers
inquiry

4439-98-9
8 suppliers
inquiry
![(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-((tert-butoxycarbonyl)amino)-5 a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate](/CAS/20200611/GIF/1392315-38-6.gif)
1392315-38-6
2 suppliers
inquiry
![tert-butyl ((1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-5a,5b,8,8,11a-pentamethyl-9-oxo-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysen-3a-yl)carbamate](/CAS/20180703/GIF/1392315-37-5.gif)