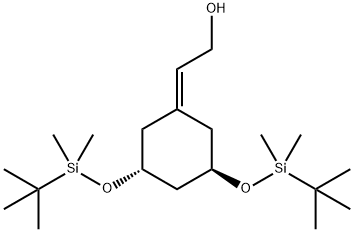
2-((3R,5R)-3,5-bis(tert-butyldiMethylsilyloxy)cyclohexylidene)ethanol synthesis
- Product Name:2-((3R,5R)-3,5-bis(tert-butyldiMethylsilyloxy)cyclohexylidene)ethanol
- CAS Number:139356-37-9
- Molecular formula:C20H42O3Si2
- Molecular Weight:386.72
![(3R-trans)-[3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]cyclohexylidene]-acetic Acid Ethyl Ester](/CAS/GIF/139356-36-8.gif)
139356-36-8
12 suppliers
$139.90/2mg

139356-37-9
44 suppliers
inquiry
Yield:139356-37-9 95%
Reaction Conditions:
with diisobutylaluminium hydride in toluene at -78; for 1 h;
Steps:
[0179] For example, compound a is esterified and the hydroxyl groups are protected to result in compound b (p-TsOH, MeOH, rt, 24 h, 92%; TBDMSCl, TEA, DMF, rt, 18 h, 70%). The thioimidazolide, c, is prepared through reaction of b with 1,1 '-thiocarbonyl-diimidazole in methylene chloride (60 h, rt, 90%). Radical deoxygenation of c with tributyltin hydride in the presence of azobisisobutyronitrile (AIBN) produces the desoxy-ester, d (Bu3SnH, AIBN, toluene, 80 °C, 2 h, 90%). Ester d is reduced to the alcohol, e (DIBAL-H, toluene, -78 °C, 2 h, 60%), which then undergoes oxidation to form cyclohexanone derivative f (saturated NaI04 in water, MeOH, 0 °C, 30 min, 78%), Reaction of f with ethyl (trimethylsilyl)acetate in the presence of lithium diisopropylamide (LDA) in THF (-78 °C, 2 h, 86%) produces the cyclohexyldiene ester, g. The latter is reduced to the allylic alcohol, h (DIBAL-H, toluene, -78 °C, 1 h, 78-95%), which is converted to chloride i by reaction with the complex made from N-chlorosuccinimide and dimethyl sulfide (-25 °C, then 0 °C, 80%). This chloride is treated with lithium diphenylphosphideO °C (-78 °C, 30 min), followed by oxidation with hydrogen peroxide, to form compound j. Compound j is partially deprotected to form compound k, which is dehydroxylated as described for compound c, to result in compound m. Compound m can react with a ketone precursor by methods described herein to form the 19-nor prodrugs of the invention.
References:
WO2011/88209,2011,A2 Location in patent:Page/Page column 59-60
![2-Cyclohexen-1-one, 5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, (5S)-](/CAS/20180527/GIF/199613-48-4.gif)
199613-48-4
0 suppliers
inquiry

139356-37-9
44 suppliers
inquiry
![(3S,5S)-3,5-Bis[[(1,1-diMethylethyl)diMethylsilyl]oxy]-1-hydroxy-cyclohexaneMethanol](/CAS/GIF/139356-34-6.gif)
139356-34-6
8 suppliers
$174.90/5mg

139356-37-9
44 suppliers
inquiry
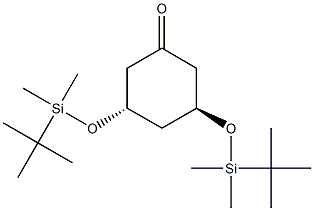
139356-35-7
6 suppliers
inquiry

139356-37-9
44 suppliers
inquiry

![Acetic acid, 2-[(3R,5R)-3,5-bis(acetyloxy)cyclohexylidene]-, ethyl ester](/CAS/20210305/GIF/332097-70-8.gif)