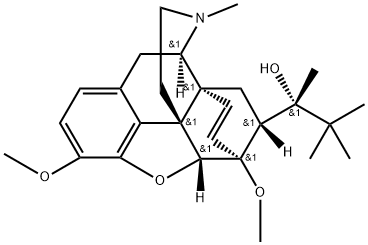
(αS,5α,7α)-α-(1,1-DiMethylethyl)-4,5-epoxy-3,6-diMethoxy-α,17-diMethyl-6,14-ethenoMorphinan-7-Methanol synthesis
- Product Name:(αS,5α,7α)-α-(1,1-DiMethylethyl)-4,5-epoxy-3,6-diMethoxy-α,17-diMethyl-6,14-ethenoMorphinan-7-Methanol
- CAS Number:13965-70-3
- Molecular formula:C27H37NO4
- Molecular Weight:439.6
Yield:13965-70-3 83%
Reaction Conditions:
in diethyl ether;diethylene glycol dimethyl ether;toluene at 0 - 10; for 1.5 h;
Steps:
III.b step b) - directly from Compound ha, no hydrogenation
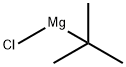
A solution of tert- Butyl magnesium chloride 1.7 M solution in diethyl ether (92.5 mL, 157.3 mmol) was diluted with toluene (70 mL) and cooled to 5 °C. Diglyme (14.35 g, 106.9 mmol) was added slowly at 5 - 10 °C. A solution of Compound Ila (10.0 g, 26.2 mmol) in a 1: 1 (w/w) toluene/diethyl ether mixture (75 mL) was added dropwise to the above mixture, maintaining internal temperature in the range of 0 - 5 °C. Stirring was continued at 5 °C for 1.5 h. The reaction was quenched with a solution of acetone in diethyl ether, followed by the addition of a saturated ammonium chloride solution. Phases were separated and the organic layer was washed with water. The toluene/ether solvent was evaporated and gradually replaced by ethanol (150 mL). The solution was heated under reflux for an hour, water (36 mL) was added, refluxed for another hour and allowed to cool to 5 °C. Precipitated solid was filtered off, the product was washed with EtOH/water 1 : 1 mixture and dried in vacuum to afford 9.56 g of Compound Ilia (83 % yield) as a white solid with an HPLC purity of 99.8 %.
References:
WO2021/151908,2021,A1 Location in patent:Page/Page column 15
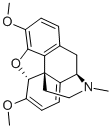
115-37-7
0 suppliers
$21.00/1mg

13965-70-3
2 suppliers
inquiry
![1-[(5alpha,7alpha)-4,5-epoxy-3,6-dimethoxy-17-methyl-6,14-ethenomorphinan-7-yl]ethanone](/CAS/GIF/15358-22-2.gif)