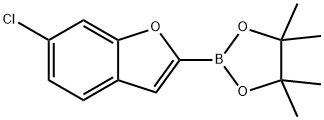
2-(6-Chlorobenzofuran-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(6-Chlorobenzofuran-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:1396751-50-0
- Molecular formula:C14H16BClO3
- Molecular Weight:278.54

151619-12-4
24 suppliers
inquiry
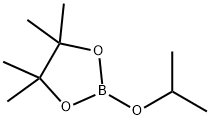
61676-62-8
312 suppliers
$11.19/5G

1396751-50-0
5 suppliers
inquiry
Yield:1396751-50-0 62%
Reaction Conditions:
Stage #1: 6-chloro benzo[b]furanwith n-butyllithium in tetrahydrofuran;hexane at -78; for 1.5 h;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -78; for 2.5 h;
Steps:
2 Step-2: Synthesis of 2-(6-chlorobenzofuran-2-yl)-4,4,5,5-tetramethyl-i,3,2- dioxaborolane (4):
To a stirred solution of 6-chlorobenzofuran 2 (0.40 g, 2.63 mmol) in THF (8 mL) was added 1.4M n-BuLi in hexane (1.90 mL, 2.63 mmol) at -78°C and stirred for 1.5 h. 2-isopropoxy- 4>4>5>5-tetramethyl-i,3,2-dioxaborolane 3 (0.98 mg, 5.26 mmol) was added dropwise at -78 °C and the reaction mixture was stirred at same temperature for 2.5h. After completion of the reaction (monitored by TLC), the reaction mixture was quenched with water (30 mL) extracted with ethyl acetate (3 c 25 mL) and washed with water (25 mL). The organic layer was separated, dried over anhydrous Na2S04, concentrated under reduced pressure. Crude compound was purified by silica gel column chromatography eluting with 10-15% ethyl acetate in n-hexane to afford 0.45 g (62% yield) of 4 as colourless oil. NMR (400 MHz, CDCl3) d: 7-53 - 7-58 (m, 2 H), 7.37 (s, 1 H), 7.22 - 7.25 (m, 1 H), 1.40 (s, 12 H).
References:
WO2020/69625,2020,A1 Location in patent:Page/Page column 22-23
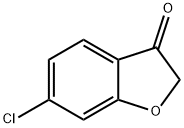
3260-78-4
115 suppliers
$50.00/1g

1396751-50-0
5 suppliers
inquiry