
4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester synthesis
- Product Name:4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester
- CAS Number:1400589-53-8
- Molecular formula:C17H22ClN5O3
- Molecular Weight:379.84
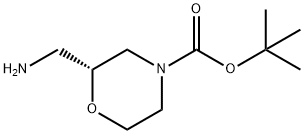
1174913-80-4
91 suppliers
$94.00/250mg
![5,7-Dichloropyrido[4,3-b]pyrazine](/CAS/20150408/GIF/1379338-74-5.gif)
1379338-74-5
59 suppliers
$60.00/5g
![4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester](/CAS/20180703/GIF/1400589-53-8.gif)
1400589-53-8
21 suppliers
$16.00/100mg
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1-methyl-pyrrolidin-2-one at 130; for 0.666667 h;microwave irradiation;
Steps:
22
Intermediate 22: 1 ,1-Dimethylethyl (2/~)-2-{r(7-chloropyridor3,4-6lpyrazin-5- yl)amino1methylV4-morpholinecarboxylate; 1 , 1-dimethylethyl (2f~)-2-(aminomethyl)-4-morpholinecarboxylate (for preparation see: J. Medicinal Chemistry, 2009, 52 (15), 4810-4819) (6g, 27.7mmol) was dissolved in N-methyl-2-pyrrolidinone (NMP) (60ml_) and to this was added DIPEA (7.27ml_, 41.6mmol) and 5,7-dichloropyrido[3,4-b]pyrazine (5.55g, 27.7mmol). This was split between 4 large microwave vials and each was heated at 130°C for 30min. They were monitored by LCMS and were given a further 10min at 130°C. The reaction mixtures were partitioned between ethyl acetate (700ml) and diluted aqueous ammonium chloride (1 litre). The aqueous was reextracted with ethyl acetate (300ml) and the combined organics were washed with aqueous ammonium chloride (500ml), dried over sodium sulfate and concentrated in vacuo to yield a crude brown oil. It was dissolved in DCM and passed through silica (70g) eluting with DCM (6 X 40ml) then 5% ethyl acetate in DCM (2 X 40ml), 10% ethyl acetate in DCM (5 X 40ml) then 15% ethyl acetate in DCM (2 X 40ml) then 20%ethyl acetate in DCM (2 X 40ml). Appropriate fractions were combined and concentrated in vacuo to yield: N8231-100-2, orange-yellow slightly gummy solid, 7.7gLCMS (Method B): Rt = 1.17min, MH+ 380
References:
WO2012/123312,2012,A1 Location in patent:Page/Page column 73-74
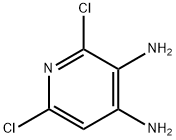
101079-63-4
110 suppliers
$25.00/100mg
![4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester](/CAS/20180703/GIF/1400589-53-8.gif)
1400589-53-8
21 suppliers
$16.00/100mg
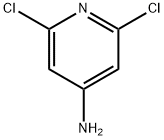
2587-02-2
292 suppliers
$12.40/250mg
![4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester](/CAS/20180703/GIF/1400589-53-8.gif)
1400589-53-8
21 suppliers
$16.00/100mg
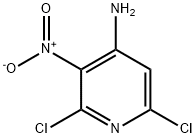
2897-43-0
169 suppliers
$7.00/250mg
![4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester](/CAS/20180703/GIF/1400589-53-8.gif)
1400589-53-8
21 suppliers
$16.00/100mg

2587-03-3
20 suppliers
inquiry
![4-Morpholinecarboxylicacid,2-[[(7-chloropyrido[3,4-b]pyrazin-5-yl)amino]methyl]-,1,1-dimethylethylester](/CAS/20180703/GIF/1400589-53-8.gif)
1400589-53-8
21 suppliers
$16.00/100mg