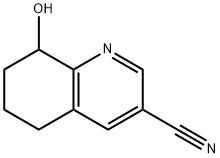
8-Hydroxy-5,6,7,8-tetrahydro-quinoline-3-carbonitrile synthesis
- Product Name:8-Hydroxy-5,6,7,8-tetrahydro-quinoline-3-carbonitrile
- CAS Number:1400683-17-1
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
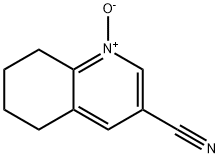
1400683-16-0
0 suppliers
inquiry

1400683-17-1
5 suppliers
inquiry
Yield:1400683-17-1 87.1%
Reaction Conditions:
Stage #1: 3-cyano-5,6,7,8-tetrahydroquinoline 1-oxidewith trifluoroacetic anhydride in acetic acid at 23; for 18 h;Cooling;
Stage #2: with sodium hydroxide in water;acetic acid; for 0.5 h;
Steps:
c
c) 8-Hydroxy-5,6,7,8-tetrahydroquinoline-3-carbonitrile To a solution of 3-cyano-5,6,7,8-tetrahydroquinoline 1-oxide (645 mg, 3.7 mmol) was added dropwise under ice cooling trifluoroacetic anhydride (6.22 g, 4.18 ml, 29.6 mmol). The light yellow solution was stirred at 23° C. for 18 h. The mixture was quenched with 1 N NaOH solution and stirred vigorously for 30 min., then extracted twice with dichloromethane, the combined organic layers were dried over Na2SO4, filtered and evaporated. The residue was purified by silica gel flash chromatography with n-heptane/ethyl acetate to give the 8-hydroxy-5,6,7,8-tetrahydroquinoline-3-carbonitrile (562 mg, 3.23 mmol, 87.1% yield) as a white solid. MS (ISP): m/z=175.1 [(M+H)+].
References:
US2012/238548,2012,A1 Location in patent:Page/Page column 41

1400683-18-2
5 suppliers
inquiry

1400683-17-1
5 suppliers
inquiry

101871-76-5
11 suppliers
inquiry

1400683-17-1
5 suppliers
inquiry

65242-27-5
24 suppliers
inquiry

1400683-17-1
5 suppliers
inquiry