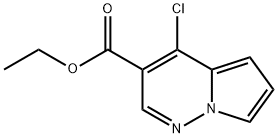
ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate synthesis
- Product Name:ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate
- CAS Number:1400688-74-5
- Molecular formula:C10H9ClN2O2
- Molecular Weight:224.64
![Pyrrolo[1,2-b]pyridazine-3-carboxylic acid, 4-hydroxy-, ethyl ester](/CAS/20210111/GIF/1260849-93-1.gif)
1260849-93-1
2 suppliers
inquiry
![ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate](/CAS/20180703/GIF/1400688-74-5.gif)
1400688-74-5
18 suppliers
inquiry
Yield:1400688-74-5 100%
Reaction Conditions:
Stage #1: ethyl 4-hydroxypyrrolo[1,2-b]pyridazine-3-carboxylatewith triethylamine;trichlorophosphate at 0 - 110; for 16 h;
Stage #2: with ethanol in dichloromethane at 0 - 20; for 1.5 h;
Stage #3: with Sodium hydrogenocarbonate in dichloromethane;lithium hydroxide monohydrate; for 1 h;
Steps:
2.5B
Step 5B: Alternative preparation of Ethyl 4-chloropyrrolo [1, 2-b] pyridazine-3- carboxylate (Preparation 2)[00162] To POCl3 (225 mL, 2.42 mmol) at 0 °C was added ethyl 4- hydroxypyrrolo[l, 2-b] pyridazine-3-carboxylate (50 g, 0.242 mmol) and the mixture was stirred until complete dissolution. At this time, triethylamine (36.8 mL, 0.266 mmol) was added dropwise and the resulting mixture was heated to 110 °C and allowed to stir for ~16h. The resulting mixture was allowed to cool and the POCI3 was removed in vacuo to afford a dark brown residue. This material was dissolved in dichloromethane and was cooled to 0 °C and diluted with 375 mL of ethanol. The resulting mixture was stirred at 0 °C for 30 min and then at rt for 1 h. The ethanol was removed under vacuum and the resulting semi-solid was dissolved indichloromethane and stirred with 10% aqueous NaHCC^ for 1 h. The mixture was filtered through celite and the phases were separated. The aqueous portion was extracted with additional dichloromethane and the combined organic layers were washed with brine, dried over Na2S04, decanted and the solvent was removed under vacuum to give the crude product. The crude material was purified by flash silica gel column chromatography to yield 47 g (86%) of a solid as the title compound. XH NMR (400MHz, CDC13) δ ppm: 1.43 (t, J=7.2, 6.8 Hz, 3 H), 4.43 (q, J=7.2, 6.8 Hz, 2 H), 6.940-6.99 (m, 2H), 7.84 (q, J=0.8, 1.6 Hz, 1 H), 8.51 (s, 1H).). 13C-NMR (400 MHz, DMS-i?) ppm: 163.30, 141.89, 138.16, 124.86, 120.69, 1 14.99, 109.90, 105.22, 61.55, 14.21. LCMS (conditions A): m/z 225. HPLC (condition B): Retention time = 3.47 min.
References:
WO2012/125887,2012,A1 Location in patent:Page/Page column 65
![Pyrrolo[1,2-b]pyridazine-3-carboxylic acid, 4-hydroxy-, ethyl ester](/CAS/20210111/GIF/1260849-93-1.gif)
1260849-93-1
2 suppliers
inquiry
![4-chloropyrrolo[1,2-b]pyridazine-3-carboxylic acid](/CAS/20180629/GIF/1400688-73-4.gif)
1400688-73-4
12 suppliers
inquiry
![ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate](/CAS/20180703/GIF/1400688-74-5.gif)
1400688-74-5
18 suppliers
inquiry
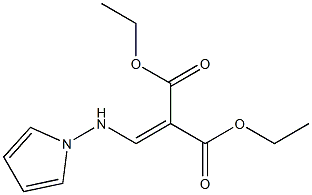
156335-36-3
1 suppliers
inquiry
![ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate](/CAS/20180703/GIF/1400688-74-5.gif)
1400688-74-5
18 suppliers
inquiry
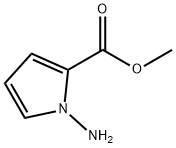
122181-85-5
50 suppliers
inquiry
![ethyl 4-chloropyrrolo[1,2-b]pyridazine-3-carboxylate](/CAS/20180703/GIF/1400688-74-5.gif)
1400688-74-5
18 suppliers
inquiry
![Pyrrolo[1,2-b]pyridazine-3-carboxylic acid, 6-chloro-4-hydroxy-, ethyl ester](/CAS/20210305/GIF/1400688-81-4.gif)