
(S)-4-benzyl-3-(5,5,5-trifluoropentanoyl)oxazolidin-2-one synthesis
- Product Name:(S)-4-benzyl-3-(5,5,5-trifluoropentanoyl)oxazolidin-2-one
- CAS Number:1401065-46-0
- Molecular formula:C15H16F3NO3
- Molecular Weight:315.29
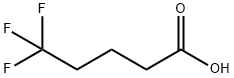
407-62-5
76 suppliers
$15.00/100mg

90719-32-7
491 suppliers
$5.00/5g

1401065-46-0
14 suppliers
inquiry
Yield:1401065-46-0 85%
Reaction Conditions:
Stage #1: 5,5,5-trifluoropentanoic acid;(S)-4-Benzyl-2-oxazolidinonewith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane; for 2 h;
Stage #2: (S)-4-Benzyl-2-oxazolidinonewith n-butyllithium in tetrahydrofuran at -78; for 0.583333 h;
Steps:
Intermediate S-1B: (4S)-4-Benzyl-3-(5,5,5-trifluoropentanoyl)-1,3-oxazolidin-2-one
Intermediate S-1B: (4S)-4-Benzyl-3-(5,5,5-trifluoropentanoyl)-1,3-oxazolidin-2-one
To a stirring solution of 5,5,5-trifluoropentanoic acid (14.76 g, 95 mmol) and DMF (0.146 mL) in DCM (50 mL) was slowly added oxalyl chloride (8.27 mL, 95 mmol).
After 2 h, the mixture was concentrated to dryness.
A separate flask was changed with (S)-4-benzyloxazolidin-2-one (16.75 g, 95 mmol) in THF (100 mL) and then cooled to -78° C.
To the solution was slowly added n-BuLi (2.5M, 37.8 mL, 95 mmol) over 10 min, stirred for 10 min, and then a solution of the above acid chloride in THF (50 mL) was slowly added over 5 min.
The mixture was stirred for 30 min, and then warmed to room temperature.
The reaction was quenched with sat aq NH4Cl.
Next, 10% aq LiCl was then added to the mixture, and the mixture was extracted with Et2O.
The organic layer was washed with sat aq NaHCO3 then with brine, dried (MgSO4), filtered and concentrated to dryness.
The residue was purified by SiO2 chromatography (ISCO, 330 g column, eluting with a gradient from 100% hexane to 100% EtOAc) to afford the product Intermediate S-1B; (25.25 g, 85%): 1H NMR (400 MHz, CDCl3) δ ppm 7.32-7.39 (2H, m), 7.30 (1H, d, J=7.05 Hz), 7.18-7.25 (2H, m), 4.64-4.74 (1H, m), 4.17-4.27 (2H, m), 3.31 (1H, dd, J=13.35, 3.27 Hz), 3.00-3.11 (2H, m), 2.79 (1H, dd, J=13.35, 9.57 Hz), 2.16-2.28 (2H, m), 1.93-2.04 (2H, m).
References:
US2014/87992,2014,A1 Location in patent:Paragraph 0268-0269
1186202-35-6
3 suppliers
inquiry

90719-32-7
491 suppliers
$5.00/5g

1401065-46-0
14 suppliers
inquiry

407-62-5
76 suppliers
$15.00/100mg

1401065-46-0
14 suppliers
inquiry