
2,5-Piperazinedione, 3-(hydroxymethyl)-6-methyl-, (3S,6R)- synthesis
- Product Name:2,5-Piperazinedione, 3-(hydroxymethyl)-6-methyl-, (3S,6R)-
- CAS Number:1403898-62-3
- Molecular formula:C6H10N2O3
- Molecular Weight:158.16
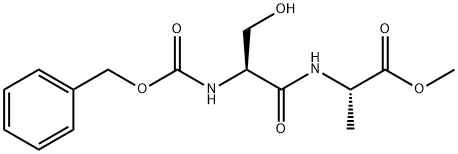
![D-Alanine, N-[(phenylmethoxy)carbonyl]-L-seryl-, methyl ester](/CAS/20200611/GIF/1403898-61-2.gif)
1403898-61-2
3 suppliers
inquiry

1403898-62-3
4 suppliers
inquiry
Yield:1403898-62-3 88%
Reaction Conditions:
with palladium 10% on activated carbon in methanol;cis-cyclohexene; for 1 h;Reflux;Inert atmosphere;
Steps:
(3S,6R)-3-(Hydroxymethyl)-6-methylpiperazine-2,5-dione
Methyl N-[(benzyloxy)carbonyl]-L-seryl-D-alaninate (44.9 g, 138.44 mmol) was dissolved in 127 methanol (140 ml) and 306 cyclohexene (90 ml, 888.54 mmol). 10% 307 Palladium on carbon (2.25 g, 10.90 mmol) was added in one portion and the resultant mixture was heated to reflux overnight. A further portion of methanol (120 ml) was added and the mixture was stirred at reflux for one hour. The reaction mixture was filtered (whilst hot) through celite and the celite was washed with hot methanol (2×300 ml). The filtrate was concentrated to give a grey semi-solid. The solid was triturated with acetonitrile (200 ml), filtered and washed with acetonitrile (100 ml). The solid was dried under vacuum at 40° C. for one hour to give 308 (3S,6R)-3-(hydroxymethyl)-6-methylpiperazine-2,5-dione (19.22 g, 88%) as a grey solid. 1H NMR (400 MHz, DMSO, 30° C.): 1.24 (3H, d), 3.52 (1H, ddd), 3.68 (1H, q), 3.74 (1H, ddd), 3.92 (1H, q), 5.09 (1H, t), 7.87 (1H, s), 8.06 (1H, s).
References:
US2019/177338,2019,A1 Location in patent:Paragraph 0348; 0527-0528
38428-20-5
2 suppliers
$28.60/50mg

1403898-62-3
4 suppliers
inquiry