
2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, phenylMethyl ester synthesis
- Product Name:2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, phenylMethyl ester
- CAS Number:140927-07-7
- Molecular formula:C14H15NO2
- Molecular Weight:229.27

50-00-0
851 suppliers
$10.00/25g
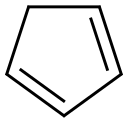
542-92-7
94 suppliers
inquiry

501-53-1
395 suppliers
$10.00/1g
![2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, phenylMethyl ester](/CAS/GIF/140927-07-7.gif)
140927-07-7
17 suppliers
inquiry
Yield:140927-07-7 21%
Reaction Conditions:
Stage #1: formaldehyd;cyclopenta-1,3-dienewith ammonium chloride in water at 20; for 36 h;
Stage #2: benzyl chloroformatewith sodium carbonate in water at 0; for 2 h;
Steps:
86
Intermediate I-40 (Benzyl 2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate)Intermediate I-39 (48 g, 0.73 mol, 1.0 eq) and ammonium chloride (116 g, 2.2 mol, 3.0 eq) were mixed in water (400 mL), and a formaldehyde solution (37% in water, 88 mL, 1.1 mol, 1.5 eq) was added. The reaction mixture was stirred at room temperature for 36 hours, neutralized with solid Na2CO3 and cooled to 0° C. To this mixture were added simultaneously benzyl chloroformate (124 g, 0.73 mol, 1.0 eq) and a solution of Na2CO3 (38.6 g, 0.364 mol, 0.5 eq) in water (400 mL) at such a rate that the addition of Na2CO3 was completed immediately after the addition of benzyl chloroformate. The reaction mixture was stirred for 2 hours at 0° C. The reaction mixture was diluted with H2O (1 L) and extracted with dichloromethane (4×1 L). The combined organic layers were dried over anhydrous Na2SO4, the solids were removed by filtration and the filtrate was concentrated. The crude reaction product was purified by silica gel chromatography to give intermediate I-40 (35g, 21%). MS (ESI): m/z 203 (M+H+).
References:
US2011/65694,2011,A1 Location in patent:Page/Page column 65
![2-Azabicyclo[2.2.1]hept-5-ene](/CAS/GIF/6671-85-8.gif)
6671-85-8
42 suppliers
$45.00/10mg

501-53-1
395 suppliers
$10.00/1g
![2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, phenylMethyl ester](/CAS/GIF/140927-07-7.gif)
140927-07-7
17 suppliers
inquiry
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)