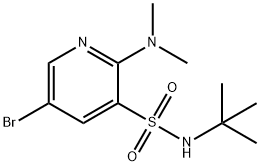
5-BROMO-N-(TERT-BUTYL)-2-(DIMETHYLAMINO)PYRIDINE-3-SULFONAMIDE synthesis
- Product Name:5-BROMO-N-(TERT-BUTYL)-2-(DIMETHYLAMINO)PYRIDINE-3-SULFONAMIDE
- CAS Number:1416336-56-5
- Molecular formula:C11H18BrN3O2S
- Molecular Weight:336.25
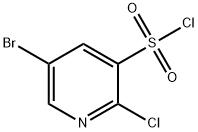
1146290-19-8
33 suppliers
$310.00/100mg

124-40-3
516 suppliers
$18.00/100ml
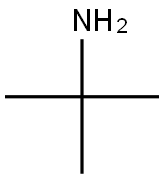
75-64-9
428 suppliers
$10.00/10g

1416336-56-5
4 suppliers
inquiry
Yield:1416336-56-5 78%
Reaction Conditions:
Stage #1: 5-bromo-2-chloro-pyridine-3-sulfonyl chloride;tert-butylaminewith pyridine in dichloromethane at 20; for 0.333333 h;
Stage #2: dimethyl amine in tetrahydrofuran at 50; for 2 h;
Steps:
128.A
Step A 5-bromo-N-(tert-butyl)-2-(dimethylamino)pyridine-3-sulfonamide[00289] A solution of 5-bromo-2-chloro-3-pyridinesulfonyl chloride (0.5 g, 1.719 mmol), pyridine (0.142 mL, 1.753 mmol) and 2-methylpropan-2-amine (0.402 mL, 7.05 mmol) in dichloromethane (DCM) (5 mL) was stirred at room temperature for 20 minutes then the mixture was evaporated, the residue dissolved in EtOAc, washed with water and brine, dried over magnesium sulfate and was evaporated again to give a yellow crystalline intermediate. It was dissolved in tetrahydrofuran (THF) (5.00 mL), 2M dimethylamine in THF (2.58 mL, 5.16 mmol) was added and the solution was heated at 50 °C for 2 hrs. The reaction mixture was then evaporated, the residue dissolved in EtOAc and water, the organic phase was separated and washed with brine, dried over magnesium sulfate and was evaporated again to give 5-bromo-N- (tert-butyl)-2-(dimethylamino)pyridine-3-sulfonamide (452 mg, 1.344 mmol, 78 % yield) as a brown solid. MS (ES+) m/z 336, 338 (M+H); 1 H NMR (400MHz ,DMSO-d6) δ = 8.38 (d, J = 2.2 Hz, 1 H), 8.23 (d, J = 2.3 Hz, 1 H), 7.67 (s, 1 H), 3.02 (s, 6 H), 1.05 (s, 9 H).
References:
WO2012/174312,2012,A2 Location in patent:Page/Page column 206-207
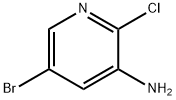
588729-99-1
301 suppliers
$3.00/1g

1416336-56-5
4 suppliers
inquiry