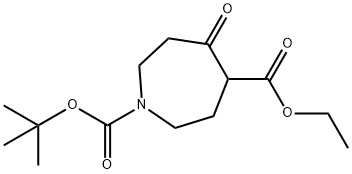
ETHYL 1-BOC-5-OXO-HEXAHYDRO-1H-AZEPINE-4-CARBOXYLATE synthesis
- Product Name:ETHYL 1-BOC-5-OXO-HEXAHYDRO-1H-AZEPINE-4-CARBOXYLATE
- CAS Number:141642-82-2
- Molecular formula:C14H23NO5
- Molecular Weight:285.34
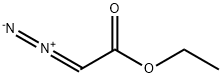
623-73-4
146 suppliers
$26.69/5g
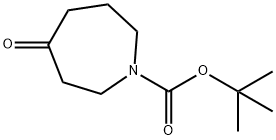
188975-88-4
263 suppliers
$6.00/250mg

141642-82-2
149 suppliers
$23.00/100mg
Yield: 85%
Reaction Conditions:
with boron trifluoride diethyl etherate in diethyl ether at -5 - 0; for 1 h;Inert atmosphere;
Steps:
5-Oxo-azepane-1,4-dicarboxylic acid 1-tert-butyl ester 4-ethyl ester:
In a dried, N2-flushed, 500-mL three-necked, round-bottomed flask equipped with a magnetic stir bar, N-BOC-piperidone 1 (20 g, 0.10 mol) and BF3·Et2O (14 mL, 0.11 mol) were diluted in Et2O (200 mL) then chilled to -5 °C. Slowly, ethyl diazoacetate (13.7 mL, 0.13 mol) was added over a period of 1 h causing extreme gas evolution. The internal temperature was maintained between -5 °C and 0 °C during the addition. The reaction was stirred for 1 h at 0 °C then slowly quenched with 30% Na2CO3 at 0 °C. The pH was adjusted to 7-8 and then H2O (30 mL) was added to the mixture. The organic layer was extracted with EtOAc (2 x 75 mL), dried with Na2SO4, filtered, and concentrated to orange oil. The crude oil was purified by column chromatography (silica gel column: 14 cm OD, 8 cm in height and eluting with 9/1 hexanes/EtOAc increasing to 7/3 hexanes/EtOAc) to provide a light yellow oil in 85% yield. MS (electrospray): C14H23NO5; m/z not found. 1H NMR (400 MHz, CDCl3): δ 4.25 - 2.03 (m, 11 H), 1.47 - 1.45 (d, J = 7.8 Hz, 9 H), 1.31 - 1.24 (m, 3 H).
References:
Dvorak, Curt A.;Rudolph, Dale A.;Nepomuceno, Diane;Dvorak, Lisa;Lord, Brian;Fraser, Ian;Bonaventure, Pascal;Lovenberg, Timothy;Carruthers, Nicholas I. [Bioorganic and Medicinal Chemistry Letters,2021,vol. 31,art. no. 127669] Location in patent:supporting information

623-73-4
146 suppliers
$26.69/5g

79099-07-3
543 suppliers
$5.00/5g

141642-82-2
149 suppliers
$23.00/100mg

24424-99-5
821 suppliers
$13.50/25G

141642-82-2
149 suppliers
$23.00/100mg
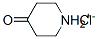
41979-39-9
117 suppliers
$15.00/10mg

141642-82-2
149 suppliers
$23.00/100mg