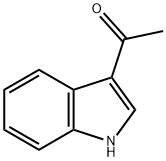
Ethanone, 1-[1-(10H-phenothiazin-10-ylcarbonyl)-1H-indol-3-yl]- synthesis
- Product Name:Ethanone, 1-[1-(10H-phenothiazin-10-ylcarbonyl)-1H-indol-3-yl]-
- CAS Number:1417560-79-2
- Molecular formula:C23H16N2O2S
- Molecular Weight:384.45
Yield:1417560-79-2 76%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 20; for 72 h;Inert atmosphere;
Steps:
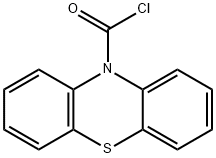
General procedure for the preparation of compounds 42, 43 (Scheme 4)
The 10H-phenothiazine-10-carbonyl chloride 44 (1.05 equiv) was dissolved in CH2Cl2 under inert atmosphere and added to a mixture of the corresponding indole derivative 45 or 46 (1 equiv), 4-dimethylaminopyridine (DMAP) (0.1 equiv) and Et3N (1.3 equiv) in CH2Cl2. The resulting mixture was stirred for 72 hours under inert atmosphere at rt, quenched with saturated aqueous NH4Cl solution, and washed several times with water and brine. The organic phase was dried over MgSO4, filtrated, and the solvent evaporated in vacuo. The obtained residue was purified by column chromatography on silica gel (EtOAc/n-heptane 5/5) to provide pure compound 42 or 43. Beigesolid; mp 178-181°C; 76% yield. IR νmax/cm-1: 1701, 1665, 1539,1448, 1354, 1309, 1261, 1192, 1151, 998, 747. 1HNMR (CDCl3, 400 MHz) δ (ppm) 2.16 (s, 3H, COCH3), 7.14-7.24 (m, 4H, ArH), 7.30-7.41 (m, 3H, ArH),7.46-7.49 (m, 2H, ArH), 7.52-7.55 (m,2H, ArH), 8.08 (d, J = 8.4 Hz, 1H, ArH), 8.28 (d, J = 7.6 Hz,1H, ArH). 13C NMR (CDCl3,100 MHz) δ (ppm) 27.2 (CH3), 113.7 (CH), 119.9 (C),122.5 (CH), 124.4 (CH), 124.9 (2CH), 125.3 (CH), 126.3 (C), 127.0 (2CH), 127.8(2CH), 127.9 (2CH), 131.7 (2C), 132.9 (CH), 136.4 (C), 138.8 (2C), 149.8 (C),193.3 (C). Anal. calcd for C23H16N2O2S 1/5 H2O: C, 71.19; H, 4.26; N, 7.22. Found: C, 71.41; H, 3.89; N, 7.03%.
References:
Abuhaie, Cristina-Maria;B?cu, Elena;Rigo, Beno?t;Gautret, Philippe;Belei, Dalila;Farce, Amaury;Dubois, Jo?lle;Ghinet, Alina [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 1,p. 147 - 152] Location in patent:supporting information