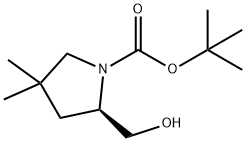
1-Pyrrolidinecarboxylic acid, 2-(hydroxyMethyl)-4,4-diMethyl-, 1,1-diMethylethyl ester, (2R)- synthesis
- Product Name:1-Pyrrolidinecarboxylic acid, 2-(hydroxyMethyl)-4,4-diMethyl-, 1,1-diMethylethyl ester, (2R)-
- CAS Number:1417743-45-3
- Molecular formula:C12H23NO3
- Molecular Weight:229.32
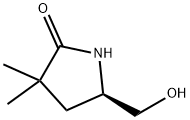
493035-98-6
5 suppliers
inquiry

1417743-45-3
5 suppliers
inquiry
Yield:1417743-45-3 435 mg
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 28.5 h;Inert atmosphere;Reflux;
Steps:
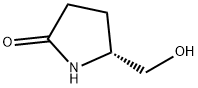
37 Description 37: (H)-feri-butyl 2-(hydroxymethyl)-4,4-dimethylpyrrolidine-1- carboxylate (D37)
To an ice cooled suspension of LiAIH4 (219 mg, 5.78 mmol) in THF (10 ml) under N2, a solution of (f?)-5-(hydroxymethyl)-3,3-dimethylpyrrolidin-2-one (D36) (690 mg, 4.82 mmol) in THF (10 ml) was added dropwise. The reaction was allowed to warm to RT over 30 min then refluxed for 5 hrs. LiAIH4 (219 mg, 5.78 mmol) was added to the mixture and the reaction stirred for 18 hrs then a further addition of LiAIH4 (219 mg, 5.78 mmol) was done and the reaction refluxed for additional 5 hrs. The reaction mixture was cooled to 0°C prior sequentially addition of water (0.87 ml), 15% NaOH (0.87 ml) and water (2.58 ml). The resulting slurry solution was filtered off; the filtrated was diluted with water (10 ml) and basified to pH~12 with Na2CO3 before adding dropwise a solution of Boc2O (1 .37 g, 6.26 mmol) in THF (10 ml). The resulting mixture was stirred for 24 hrs then extracted with EtOAc (3x30ml). The organic phases were washed with NaCI sat., dried onto Na2SO4 and evaporated in vacuo to afford the title compound (D37) (435 mg). MS: (ES/+) m/z: 252.2 [MH+Na+] C12H23NO3 requires 229.17 1 H NMR (400MHz, CHCI3-d) δ (ppm): 5.25 - 5.09 (m, 1 H), 4.13 - 3.99 (m, 1 H), 3.62 (d, J = 7.9 Hz, 2 H), 3.39 - 3.23 (m, 1 H), 3.08 - 2.92 (m, 1 H), 1 .87 - 1 .73 (m, 1 H), 1 .49 (s, 9 H), 1 .38 - 1 .25 (m, 1 H), 1 .09 (s, 3 H), 1 .03 (s, 3 H)
References:
WO2013/4290,2013,A1 Location in patent:Page/Page column 60; 61
1417743-49-7
19 suppliers
$137.00/50mg

1417743-45-3
5 suppliers
inquiry

493035-98-6
5 suppliers
inquiry

24424-99-5
822 suppliers
$13.50/25G

1417743-45-3
5 suppliers
inquiry
66673-40-3
228 suppliers
$5.00/250mg

1417743-45-3
5 suppliers
inquiry
![3H,5H-Pyrrolo[1,2-c]oxazol-5-one, tetrahydro-3,3,6,6-tetramethyl-, (7aR)-](/CAS/20210305/GIF/1417743-44-2.gif)
1417743-44-2
0 suppliers
inquiry

1417743-45-3
5 suppliers
inquiry