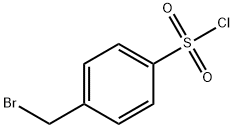
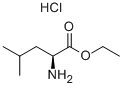
ETHYL (2S)-2-(([4-(BROMOMETHYL)PHENYL]SULFONYL)AMINO)-4-METHYLPENTANOATE synthesis
- Product Name:ETHYL (2S)-2-(([4-(BROMOMETHYL)PHENYL]SULFONYL)AMINO)-4-METHYLPENTANOATE
- CAS Number:141834-14-2
- Molecular formula:C15H22BrNO4S
- Molecular Weight:392.31
Yield:141834-14-2 85%
Reaction Conditions:
with triethylamine in tetrahydrofuran;
Steps:
1.b (b)
(b) N-4-Bromomethylphenylsulphonyl-L-leucine ethyl ester L-leucine ethyl ester hydrochloride (75.0 g. 0.403 mol) was suspended in THF (300 ml) at 0° C., and triethylamine (67 ml, 0.484 mol) added slowly. After stirring for 15 mins a solution of 4-bromomethylphenylsulphonyl chloride (108.4 g, 0.403 mol) in THF (100 ml) was added via cannular. The reaction mixture was allowed to stir overnight at ambient temperature. The solvent was removed under low pressure and the organics were extracted into ethyl acetate (200 ml) and washed with water (100 ml) and brine (100 ml). The organic portion was dried, filtered and the solvent evaporated under low pressure. The product was recrystallized from DIPE (500 ml) to give N-4-bromomethylphenylsulphonyl-L-leucine ethyl ester (134.0 g, 85%) as a white crystalline solid. δH 7.84 (2H, d, J 8.3 Hz), 7.52 (2H, d, J 8.3 Hz), 5.06 (1H, d, J 10.1 Hz), (2H, s), 3.97-3.82 (3H, m), 1.85-1.79 (1H, m), 1.49 (2H, t, J 7.1 Hz), 1.08 t, J 7.1 Hz), 0.92 (3H, d, J 6.7 Hz), 0.91 (3H, d, J 6.5 Hz).
References:
US5516783,1996,A
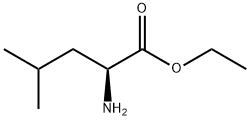
2743-60-4
20 suppliers
inquiry
![ETHYL (2S)-2-(([4-(BROMOMETHYL)PHENYL]SULFONYL)AMINO)-4-METHYLPENTANOATE](/StructureFile/ChemBookStructure9/GIF/CB6334440.gif)
141834-14-2
6 suppliers
inquiry