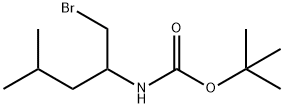
Carbamic acid, [1-(bromomethyl)-3-methylbutyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [1-(bromomethyl)-3-methylbutyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:142121-32-2
- Molecular formula:C11H22BrNO2
- Molecular Weight:280.2
Yield:142121-32-2 68%
Reaction Conditions:
with triphenylphosphine in hexane;4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran;ethyl acetate;
Steps:
33.a (a)
(a) N-tert-Butoxycarbonyl-1-bromo-2-amino-4-methylpentane A stirred solution of N-tert-butoxycarbonyl-2-aminopentan-1-ol (2.0 g, 9.2 mmol) in dry DCM (30 ml) at 0° C. was treated with tetrabromomethane (6.1 g, 18.4 mmol) followed by triphenylphosphine (4.84 g, 18.4 mmol). The clear reaction mixture immediately changed to yellow. After 30 min. the solvent was removed under reduced pressure and the residue was purified by column chromatography (flash silica gel; 0-40% ethyl acetate in hexane) to give N-tert-butoxycarbonyl-1-bromo-2-amino-4-methylpentane (1.76 g, 68%) as a colourless oil. deltaH 4.69 (1H, br d, J 8.0 Hz), 3.83 (1H, m), 3.55 (1H, dd, J 10.2, 3.9 Hz), 3.42 (1H, dd, J 10.1, 3.3 Hz), 1.61 (1H, m), 1.48-1.33 (2H, m), 1.40 (9H, s), 0.89 (6H, d, J 6.8 Hz).
References:
US5180723,1993,A