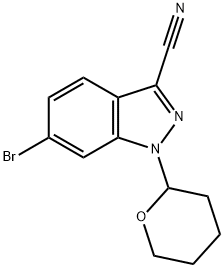
6-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-Indazole-3-carbonitrile synthesis
- Product Name:6-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-Indazole-3-carbonitrile
- CAS Number:1421503-37-8
- Molecular formula:C13H12BrN3O
- Molecular Weight:306.16
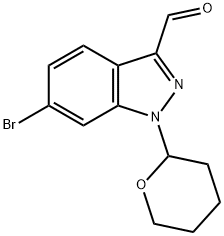
1421503-36-7
2 suppliers
inquiry

1421503-37-8
7 suppliers
inquiry
Yield:1421503-37-8 99%
Reaction Conditions:
Stage #1: 6-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole-3-carbaldehydewith hydroxylamine hydrochloride;triethylamine in acetonitrile at 60; for 3 h;
Stage #2: with trifluoroacetic anhydride in acetonitrile at 0 - 20; for 2 h;
Steps:
2 Preparation 26-Bromo-1-(tetrahvdro-pyran-2-yl)-1 H-indazole-3-carbonitrile
Preparation 26-Bromo-1-(tetrahvdro-pyran-2-yl)-1 H-indazole-3-carbonitrileTo a solution of 6-bromo-1 -(tetrahydro-pyran-2-yl)-1 H-indazole-3-carbaldehyde (Preparation 1 , 60g, 194mmol) in MeCN (1 .5L) was added triethylamine (68.5ml_, 485mmol) and hydroxylamine hydrochloride (20g, 291 mmol). The reaction was heated at 60°C for 3 hours. The reaction was cooled to 0°C, further triethylamine (220ml_, 1.55mol) was added and TFAA (109ml_, 776mmol) was added dropwise. The reaction was allowed to warm to room temperature and stirred for 2 hours. Water (2L) was added to the reaction mixture and the resulting solid was collected by filtration. The solid was dissolved in DCM (1 L) and the resulting solution was washed with water (2 x 500ml_). The organic layer was dried over MgS04 and concentrated in vacuo to give the title compound as a white solid (58.59g) in a 99% yield.1H NMR (400 MHz, CDCI3) δ ppm 1 .71 -1.80 (m, 3H), 2.08-2.18 (m, 2H), 2.43-2.50 (m, 1 H), 3.73-3.79 (m, 1 H), 3.92-3.96 (m, 1 H), 5.77 (dd, 1 H), 7.47 (dd, 1 H), 7.69 (d, 1 H), 7.93 (d, 1 H).
References:
WO2013/14567,2013,A1 Location in patent:Page/Page column 65
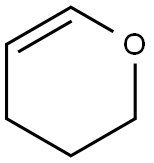
110-87-2
522 suppliers
$10.00/1g

1421503-37-8
7 suppliers
inquiry
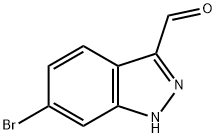
885271-72-7
172 suppliers
$40.00/250mg

1421503-37-8
7 suppliers
inquiry