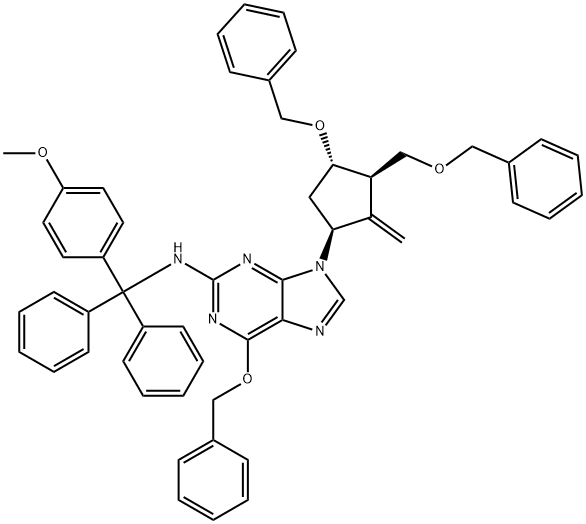
6-(Benzyloxy)-9-((1S,3S)-4-(benzyloxy)-3-((benzyloxy)methyl)-2-methylenecyclopentyl)-N-((4-methoxyphenyl)diphenylmethyl)-9H-purin-2-amine synthesis
- Product Name:6-(Benzyloxy)-9-((1S,3S)-4-(benzyloxy)-3-((benzyloxy)methyl)-2-methylenecyclopentyl)-N-((4-methoxyphenyl)diphenylmethyl)-9H-purin-2-amine
- CAS Number:142217-80-9
- Molecular formula:C53H49N5O4
- Molecular Weight:819.99
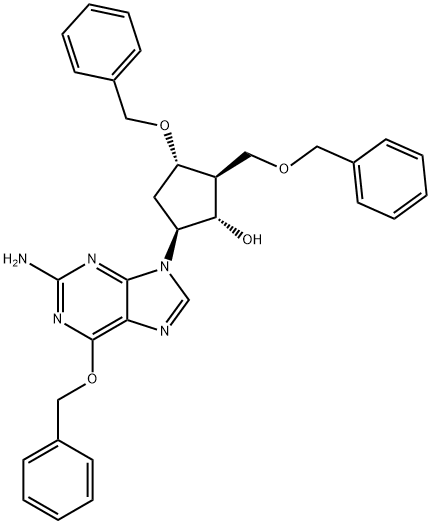
![(2R,3S,5S)-3-(Benzyloxy)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-(benzyloxy)-9H-purin-9-yl]-2-(benzyloxymethyl)cyclopentanone](/CAS/GIF/142217-79-6.gif)
142217-79-6
60 suppliers
$498.80/5MG

142217-80-9
192 suppliers
$44.00/100mg
Yield:142217-80-9 88%
Reaction Conditions:
with hydrogenchloride;titanium tetrachloride;zinc in tetrahydrofuran;dichloromethane;water at -30 - 20;Inert atmosphere;Sealed tube;Molecular sieve;
Steps:
2.1 Eample 1
Step (1) Preparation of intermediate IX, in a sealed nitrogen-filled 20L reaction bottle, 3.8kg of zinc powder and 10wt% hydrochloric acid (12.5L) were sequentially added, stirred at room temperature for 10-15min, suction filtered, washed with purified water to neutral, Wash with 3.0L ethanol, 4.5L tetrahydrofuranPolyester, 40 ± 2 ° C vacuum drying 4-4.5h to obtain about 3kg activated zinc powder. The activated zinc powder is sealed and stored under vacuum.In a 100L reactor, evacuate, replace N2, add 40L of dry (type A molecular sieve drying) tetrahydrofuran, add dry activated 3kg zinc powder and 1.3L of dibromomethane with stirring, and cool the mixture to -70~-55 °C And then drop1140 ml of titanium tetrachloride was added dropwise, stirred for 1 h, then warmed up, reacted for 48 ± 0.5 h, and sealed for use.The olefination reagent was cooled to -20-30 ° C, and 1.2 kg of a solution of Intermediate VIII in 20 L of dichloromethane was added dropwise. After the addition, the temperature was raised to 15-20 ° C and the reaction was stirred for 180 ± 10 min. TLC was used to detect the endpoint of the reaction. The reacted solution was pumped into a 200 L reaction vessel (30 L of dichloromethane, 100 L of purified water, 10 kg of sodium hydrogencarbonate mixed system) and stirred for 60-70 min. The mixture was washed with a stainless steel centrifuge, and the filter cake was washed twice with dichloromethane (16L). The combined filtrates were collected and separated, and the aqueous phase was extracted with dichloromethane (16L×2).Concentrate to dryness in vacuo at 25-30 ° C to give 6-benzyloxy-9-((1S,3R,3S)-4-benzyloxy-3-benzyloxymethyl-2-methylenecyclopentyl) -N-((4-methoxybenzene)The product is a yellow powder of diphenylmethyl)-9H-indol-2-amine, intermediate IX, a single step yield of 88% and a purity of 93.6%.
References:
Hubei Guangji Pharmaceutical Co., Ltd.;Lu Zhengdong;An Jing;Guo Shaozhi;Zheng Zhichang;Wang Liu;Li Yanhua;Ke Boxiong CN108148061, 2018, A Location in patent:Paragraph 0028; 0029; 0030; 0031; 0032; 0033; 0034
![(2R,3S,5S)-3-(Benzyloxy)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-2-(benzyloxymethyl)cyclopentanol](/CAS/GIF/142217-78-5.gif)
142217-78-5
192 suppliers
$160.00/1g

142217-80-9
192 suppliers
$44.00/100mg
142217-77-4
191 suppliers
$37.00/250mg

142217-80-9
192 suppliers
$44.00/100mg
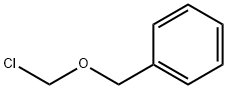
3587-60-8
317 suppliers
$10.00/1g

142217-80-9
192 suppliers
$44.00/100mg
![Benzene, [(2,4-cyclopentadien-1-ylmethoxy)methyl]-](/CAS/20200119/GIF/39939-07-6.gif)
39939-07-6
2 suppliers
inquiry

142217-80-9
192 suppliers
$44.00/100mg