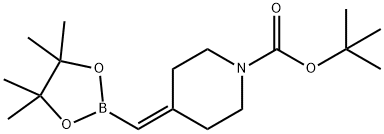
tert-Butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate
- CAS Number:1425970-61-1
- Molecular formula:C17H30BNO4
- Molecular Weight:323.24
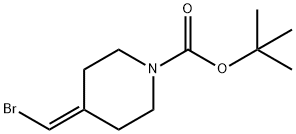
1020329-80-9
61 suppliers
$45.00/10mg

73183-34-3
564 suppliers
$6.00/5g

1425970-61-1
49 suppliers
$37.00/100mg
Yield: 43%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium acetate;triphenylphosphine in 1,4-dioxaneReflux;
Steps:
viii.c (c) tert-Butyl 4-((4, 4,5, 5-tetramethyl- 1,3, 2-dioxaborolan-2-yI)methylene)piperidine- 1-carboxylate (122)
To a solution of tert-butyl 4-(bromomethylene)piperidine-1-carboxylate 121 (3.0 g, 10.86 mmol) in 1 ,4-dioxane (30 mL) was added 4,4,4’,4’,5,5,5’,5’-octamethyl-2,2’-bi(1 ,3,2- dioxaborolane) (4.2 g, 16.29 mmol), KOAc (2.1 g, 21.72 mmol), PPh3 (171 mg, 0.652 mmol) and Pd2(dba)3 (298 mg, 0.326 mmol). The reaction was heated at reflux overnight thencooled to room temperature and the solids removed by filtration through Celite. The filtrate was concentrated and the residue partitioned between DCM (20 mL) and water (20 mL) and the aqueous phase extracted with DCM (3 x 10 mL). The combined organic extracts were washed with brine (3 x 10 mL), dried (Na2SO4), filtered and concentrated. The residue obtained was purified by column chromatography (EtOAc/petroleum ether=1/20 v/v) to givethe title compound (1.52 g, 43%) as an off-white solid. 1H NMR (400MHz, ODd3) 65.14 (s,1 H), 3.45-3.41 (m, 4H), 2.58 (t, J = 5.6 Hz, 2H), 2.24 (t, J = 5.6 Hz, 2H), 1.45 (s, 9H), 1.25 (s,12H); LCMS RT 3.31 mm; m/z 346.2 [M+Na].
References:
CTXT PTY LIMITED;BERGMAN, Ylva Elisabet;CAMERINO, Michelle Ang;STUPPLE, Paul Anthony WO2017/153520, 2017, A1 Location in patent:Page/Page column 56
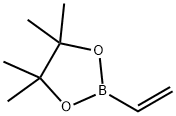
75927-49-0
206 suppliers
$6.00/1g

159635-49-1
247 suppliers
$5.00/250mg

1425970-61-1
49 suppliers
$37.00/100mg

79099-07-3
543 suppliers
$5.00/5g
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
92 suppliers
$22.00/1g

1425970-61-1
49 suppliers
$37.00/100mg

79099-07-3
543 suppliers
$5.00/5g

1425970-61-1
49 suppliers
$37.00/100mg
305794-65-4
11 suppliers
inquiry

1425970-61-1
49 suppliers
$37.00/100mg