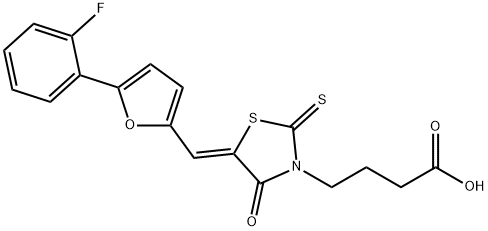
4-{5-[5-(2-Fluoro-phenyl)-furan-2-ylmethylene]-4-oxo-2-thioxo-thiazolidin-3-yl}-butyric acid synthesis
- Product Name:4-{5-[5-(2-Fluoro-phenyl)-furan-2-ylmethylene]-4-oxo-2-thioxo-thiazolidin-3-yl}-butyric acid
- CAS Number:1427538-39-3
- Molecular formula:C18H14FNO4S2
- Molecular Weight:391.44

18623-60-4
42 suppliers
$65.00/50mg
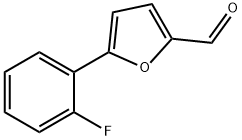
380566-25-6
50 suppliers
$45.00/100mg
![4-{5-[5-(2-Fluoro-phenyl)-furan-2-ylmethylene]-4-oxo-2-thioxo-thiazolidin-3-yl}-butyric acid](/CAS/20180702/GIF/1427538-39-3.gif)
1427538-39-3
4 suppliers
inquiry
Yield:1427538-39-3 81%
Reaction Conditions:
with piperidine in ethanol;Reflux;
Steps:
1 Synthesis of compounds 1c-40c
General procedure: The suspension of 2-thioxo-1,3-thiazolidin-4-one (0.01 mol) 1a-40a in ethanol (50 mL) was mixed under stirring with a solution of aldehyde (0.011 mol) 1b-40b followed by the addition 3 drops of piperidine under the reflux conditions. The resulting mixture was heated under reflux until complete disappearance of 2-thioxo-1,3-thiazolidin-4-one, TLC control CH3OH - EtOAc 1:9. The reaction mixture was diluted with water (75 mL) and filtrated. The solid residue was recrystallized from a mixture of IPA/DMF.
References:
Volynets, Galyna P.;Bdzhola, Volodymyr G.;Golub, Andriy G.;Synyugin, Anatoliy R.;Chekanov, Maksym A.;Kukharenko, Oleksandr P.;Yarmoluk, Sergiy M. [European Journal of Medicinal Chemistry,2013,vol. 61,p. 104 - 115]