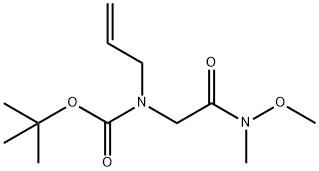
tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate synthesis
- Product Name:tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate
- CAS Number:1429646-79-6
- Molecular formula:C12H22N2O4
- Molecular Weight:258.3141

6638-79-5
559 suppliers
$6.00/25g
aMino}acetic acid](/CAS/20150408/GIF/145618-68-4.gif)
145618-68-4
19 suppliers
$65.00/50mg
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry
Yield:1429646-79-6 54%
Reaction Conditions:
Stage #1: 2-(allyl(tert-butoxycarbonyl)amino)acetic acidwith pivaloyl chloride;triethylamine in tetrahydrofuran at 0 - 20; for 3 h;
Stage #2: N,O-dimethylhydroxylamine*hydrochloridewith triethylamine in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
12 tert-Butyl N-allyl-N-[2-(methox methyl)amino)-2-oxo-ethyl]carbamate
2-(Allyl(tert-butoxycarbonyl)amino)acetic acid (49.6 g, 156 mmol) is added to tetrahydrofuran (600 mL) at 0 °C followed by the addition of triethylamine (36.3 g, 359 mmol) and pivaloyl chloride (31 g, 353 mmol). The reaction is stirred at room temperature for 3 hours and then cooled to 0 °C. N,0-dimethylhydroxylamine hydrochloride (28 g, 283 mmol), triethylamine (33 mL, 237 mmol) and tetrahydrofuran (400 mL) are then added. The ice bath is removed and the reaction stirred at room temperature for 3 hours and concentrated under reduced pressure. The resulting solid is dissolved in water and extracted with ethyl acetate. The organic phases are combined, dried over sodium sulfate, filtered, and the solvent removed under reduced pressure to give a residue. The residue is purified by silica gel column chromatography, eluting with 0-50% gradient of acetone in hexanes to give the title compound (32 g, 54%). 1H NMR(CDCI3) mixture of two rotamers (60:40) δ 1.42, 1.44 (s, 9H), 3.16, 3.17 (s, 3H), 3.66, 3.69 (s, 3H), 3.88-3.98 (m, 2H), 4.01, 4.11 (s, 2H), 5.10-5.18 (m, 2H), 5.73-5.85 (m, 1H)
References:
WO2014/66132,2014,A1 Location in patent:Page/Page column 21-22

6638-79-5
559 suppliers
$6.00/25g
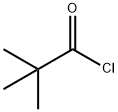
3282-30-2
361 suppliers
$19.85/100ml
aMino}acetic acid](/CAS/20150408/GIF/145618-68-4.gif)
145618-68-4
19 suppliers
$65.00/50mg
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry
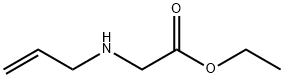
3182-79-4
12 suppliers
inquiry
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry
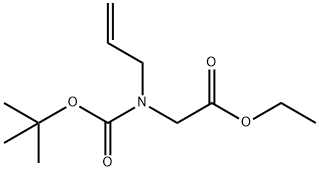
251948-87-5
11 suppliers
inquiry
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry

24424-99-5
823 suppliers
$13.50/25G
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry