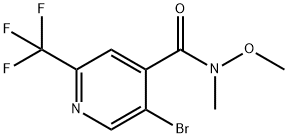
4-Pyridinecarboxamide, 5-bromo-N-methoxy-N-methyl-2-(trifluoromethyl)- synthesis
- Product Name:4-Pyridinecarboxamide, 5-bromo-N-methoxy-N-methyl-2-(trifluoromethyl)-
- CAS Number:1432054-93-7
- Molecular formula:C9H8BrF3N2O2
- Molecular Weight:313.07
944904-60-3
36 suppliers
$80.00/1g

6638-79-5
555 suppliers
$6.00/25g

1432054-93-7
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 0; for 2.16667 h;
Steps:
1 Step 1:
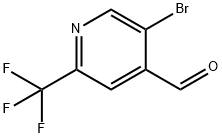
To 5-bromo-2-(trifluoromethyl)isonicotinic acid (20 g, 74.1 mmol), N,O-dimethylhydroxylamine hydrochloride (10.84 g, 111 mmol), and DIPEA (38.8 mL, 222 mmol) in DMF (100 mL) was added 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane 2,4,6-trioxide (53.4 mL, 89 mmol) at 0° C. by a dropping funnel over a period of 10 minutes. The reaction was stirred for 2 hours. The reaction was concentrated to half the amount and was diluted with EtOAc. The organic was partitioned with satd. NH4Cl and then with brine. The organic was dried over Na2SO4, filtered and concentrated in vacuo. 5-bromo-N-methoxy-N-methyl-2-(trifluoromethyl)pyridine-4-carboxamide (21 g, 67.1 mmol) was carried forward as a crude oil. 1H NMR (500 MHz, CDCl3) δ8.90 (s, 1H), 7.63 (s, 1H), 3.44 (s, 3H), 3.55 (s, 3H).
References:
US2013/109649,2013,A1 Location in patent:Paragraph 0215; 0217

6638-79-5
555 suppliers
$6.00/25g

749875-16-9
90 suppliers
$26.00/100mg

1432054-93-7
2 suppliers
inquiry