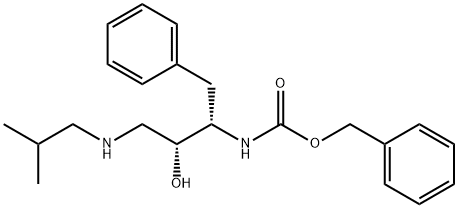
[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester synthesis
- Product Name:[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester
- CAS Number:143224-62-8
- Molecular formula:C22H30N2O3
- Molecular Weight:370.49
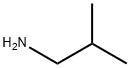
78-81-9
188 suppliers
$23.80/250ml

128018-44-0
33 suppliers
inquiry
![[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester](/CAS/20180808/GIF/143224-62-8.gif)
143224-62-8
13 suppliers
inquiry
Yield:143224-62-8 92%
Reaction Conditions:
in isopropyl alcohol; for 1.25 h;Heating / reflux;
Steps:
27.C EXAMPLE 27 Preparation of N-[3(S)-benzyloxycarbonylamino-2(R)-hydroxy-4-phenyl]N-isobutylamine; Part C
N-[3(S)-benzyloxycarbonylamino-2(R)-hydroxy-4-phenyl]N-isobutylamine
EXAMPLE 27 Preparation of N-[3(S)-benzyloxycarbonylamino-2(R)-hydroxy-4-phenyl]N-isobutylamine; Part C N-[3(S)-benzyloxycarbonylamino-2(R)-hydroxy-4-phenyl]N-isobutylamine A solution of N-benzylcarbonyl-3(S)-amino-1,2-(S)-epoxy-4-phenyl butane (50.0 g, 0.168 mol) and isobutylamine (246 g, 3.24 mol, 20 equivalents) in 650 ML of isopropyl alcohol was heated to reflux for 1.25 hours.. The solution was cooled to room temperature, concentrated in vacuo and then poured into 1 L of stirring hexane whereupon the product crystallized from solution.. The product was isolated by filtration and air dried to give 57.56 g, 92% of N[3(S)-benzyloxycarbonylamino-2(R)-hydroxy-4-phenyl]-N-isobutylamine, mp 108.0-109.5° C., MH+ m/z=371.
References:
US6388132,2002,B1 Location in patent:Page column 51-52
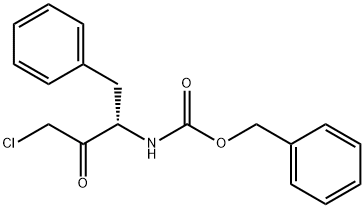
26049-94-5
98 suppliers
$35.00/250MG
![[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester](/CAS/20180808/GIF/143224-62-8.gif)
143224-62-8
13 suppliers
inquiry
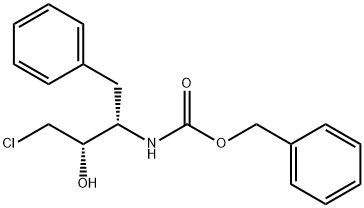
128018-43-9
25 suppliers
inquiry
![[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester](/CAS/20180808/GIF/143224-62-8.gif)
143224-62-8
13 suppliers
inquiry
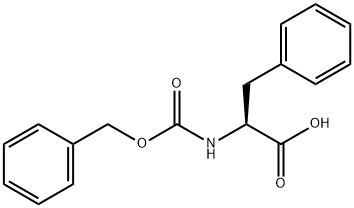
1161-13-3
368 suppliers
$5.00/5g
![[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester](/CAS/20180808/GIF/143224-62-8.gif)
143224-62-8
13 suppliers
inquiry

15196-02-8
6 suppliers
inquiry
![[(1S,2R)-2-hydroxy-3-[(2-methylpropyl)amino]-1-(phenylmethyl)propyl]-phenylmethyl ester](/CAS/20180808/GIF/143224-62-8.gif)
143224-62-8
13 suppliers
inquiry