2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethan-1-ol synthesis
- Product Name:2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethan-1-ol
- CAS Number:1434652-62-6
- Molecular formula:C14H20O4
- Molecular Weight:252.3062
Yield:1434652-62-6 30%
Reaction Conditions:
with aluminium(III) triflate at 20; for 3 h;
Steps:
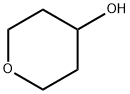
1.2 2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethanol 1d
2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethanol 1d
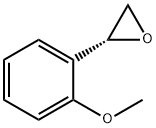
(2-methoxyphenyl)ethylene oxide 1b (26.0 g, 173.0 mmol) was added into tetrahydro-2H-pyran-4-ol 1c (53.1 g, 519.7 mmol) and aluminum trifluoromethanesulfonate (4.10 g, 8.65 mmol) with stirring.
The mixture was reacted at room temperature for 3 h.
200 mL dichloromethane and 200 mL water was added into the reaction mixture and liquid separation was conducted.
The organic phase was concentrated under reduced pressure.
The residue was purified by silica gel column chromatography (eluent: A system), to give (2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethanol 1d (13.0 g, white solid), yield: 30%.
1d 1H NMR (400 MHz, CDCl3) δ7.42 (d, J=8.0 Hz, 1H), 7.26 (t, J=7.2 Hz, 1H), 6.98 (t, J=7.2 Hz, 1H), 6.87 (d, J=8.0 Hz, 1H), 5.07 (dd, J=8.0, 4.0 Hz, 1H), 3.87-4.00 (m, 2H),3.83 (s, 3H), 3.62-3. 72 (m, 1H), 3.46-3.58 (m, 2H), 3.32-3.43 (m, 2H), 2.35-2. (m, 1H), 1.99-2.03 (m, 1H), 1.77-1.80 (m, 1H), 1.60-1.70 (m, 2H).
References:
US2020/102322,2020,A1 Location in patent:Paragraph 0122; 0125-0127