
Benzenemethanamine, 4-(1,1-dimethylethoxy)-.alpha.-methyl- synthesis
- Product Name:Benzenemethanamine, 4-(1,1-dimethylethoxy)-.alpha.-methyl-
- CAS Number:143589-82-6
- Molecular formula:C12H19NO
- Molecular Weight:193.29
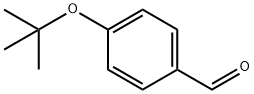
57699-45-3
129 suppliers
$60.00/2g

143589-82-6
1 suppliers
inquiry
Yield:143589-82-6 71%
Reaction Conditions:
with lithium hexamethyl-disilazane;methylmagnesium bromide in tetrahydrofuran;
Steps:
1.a Preparation of 2-[3-benzyl-5-(1-alanylamino-ethyl)-2,3,6,7-tetrahydro-1H-2-oxo-azepin-1-yl]-1-oxo-propyl-valinyl-valine methyl ester
a.) 1-(4-t-butyloxy-phenyl)-ethylamine (1) To a stirred solution of p-t-butyloxybenzaldehyde (45 g, 250 mmol) in dry THF (100 mL) under Ar at 0° C. was added a solution of lithium hexamethyldisilazane (280 mL, 1N in THF). The reaction was allowed to warm to room temperature and stirred for 15 min. A solution of methylmagnesium bromide (170 mL, 3N in ether) was next added and the reaction heated at reflux, under argon, for 2 days. The reaction was slowly poured into cold saturated ammonium chloride (500 mL) with swirling, extracted with ether (2*300 mL), washed with brine, dried over sodium sulfate and evaporated to dryness. Short path distillation under high vacuum (bp 100° C., 0.005 mm Hg) afforded product as a clear liquid (32.3 g, 71%): GC RT 6.88 min. (HP 530 mμ*20 m methylsilicone column, He carrier flow rate 20 mL/min., 100° C. init. temp., 3 min. init. time, 10° C./min. rate, 180° C. final temp., 1 min. final time); 1 H NMR (CDCl3) δ1.3 (3H, d, J=6 Hz), 1.45 (2H, s), 4.05 (1H, q), 7.1 (4H, q).
References:
US5438118,1995,A
![1-[4-(1,1-DIMETHYLETHOXY)PHENYL]-ETHANONE](/CAS/GIF/4074-63-9.gif)
4074-63-9
15 suppliers
inquiry

143589-82-6
1 suppliers
inquiry

75-16-1
264 suppliers
$12.00/10ml

57699-45-3
129 suppliers
$60.00/2g

143589-82-6
1 suppliers
inquiry