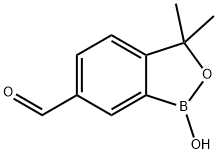
1,3-dihydro-1-hydroxy-3,3-dimethyl-2,1-benzoxaborole-6-carboxaldehyde synthesis
- Product Name:1,3-dihydro-1-hydroxy-3,3-dimethyl-2,1-benzoxaborole-6-carboxaldehyde
- CAS Number:1437051-71-2
- Molecular formula:C10H11BO3
- Molecular Weight:190
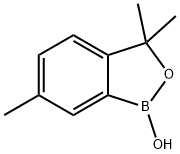
1437051-62-1
8 suppliers
inquiry

1437051-71-2
11 suppliers
inquiry
Yield:1437051-71-2 65.4%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane at 20; for 16 h;Reflux;
Steps:
2.26b.6 Step 6:
Step 6:
Preparation of 1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbaldehyde
To a solution of 3,3,6-trimethylbenzo[c][1,2]oxaborol-1(3H)-ol (6.87 g, 39 mmol) in CCl4 (100 mL) at rt was added benzoyl peroxide (0.944 g, 3.9 mmol) followed by NBS (13.9 g, 78 mmol).
The reaction mixture was refluxed for 16 h, cooled to rt and treated with Na2CO3.
The aqueous layer was acidified with 3 N HCl to pH of 3 and extracted with EA.
The organic layer was washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure.
The residue was purified by column chromatography over silica gel eluted with DCM-MeOH (20:1) to give 1-hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborole-6-carbaldehyde (4.85 g; yield 65.4%) as a pale yellow solid. 1H NMR (500 MHz, CDCl3) δ 10.06 (s, 1H), 8.15 (s, 1H), 8.01 (d, J=8.0 Hz, 1H), 7.42 (d, J=8.0 Hz, 1H), 1.58 (s, 6H) ppm. MS: m/z=191.1 (M+1, ESI+).
References:
US2013/131017,2013,A1 Location in patent:Paragraph 0409
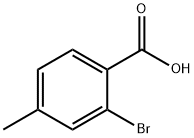
7697-27-0
204 suppliers
$6.00/1g

1437051-71-2
11 suppliers
inquiry
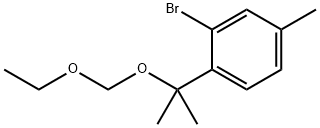
1437053-60-5
4 suppliers
inquiry

1437051-71-2
11 suppliers
inquiry
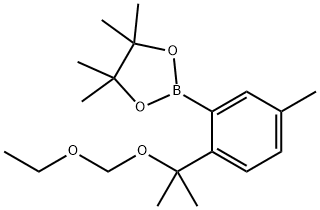
1437053-63-8
1 suppliers
inquiry

1437051-71-2
11 suppliers
inquiry
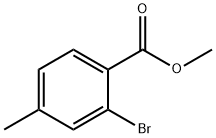
87808-49-9
114 suppliers
$8.00/1g

1437051-71-2
11 suppliers
inquiry