
3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine- 5-carboxylic acid synthesis
- Product Name:3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine- 5-carboxylic acid
- CAS Number:1437323-26-6
- Molecular formula:C14H13FN2O4
- Molecular Weight:292.26
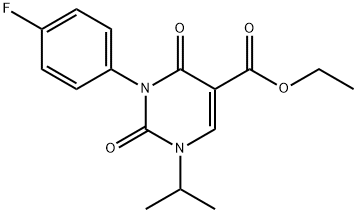
1437323-27-7
7 suppliers
inquiry

1437323-26-6
22 suppliers
inquiry
Yield: 84%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;water at 70; for 45 h;
Steps:
5.1.10 3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid (7b)
4M HCl in 1,4-dioxane (30mL, 120mmol) and water (10mL) were added to ethyl 3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (6b) (4.97g, 15.5mmol), and the reaction mixture was stirred at 70°C for 45h. The reaction mixture was cooled to room temperature, then concentrated. The residue was poured into water and the precipitate was collected by filtration, rinsed with water and dried to afford the title compound 7b as a white solid (3.81g, 13.0mmol, 84%). 1H NMR (500MHz, CDCl3): δ=1.47 (d, J=6.7Hz, 6H), 4.94 (spt, J=6.8Hz, 1H), 7.22 (d, J=6.7Hz, 4H), 8.57 (s, 1H), 12.34 (br s, 1H); 13C NMR (126MHz, CDCl3): δ=21.6, 51.2, 102.1, 116.9 (d, JCF=23.9Hz), 129.4, 129.9 (d, JCF=10.1Hz), 148.1, 150.0, 163.0 (d, JCF=250.7Hz), 163.3, 165.0; HRMS-ESI m/z [M+H]+ calcd. for C14H14FN2O4+: 293.0932, found: 293.0924.
References:
Inoue, Satoshi;Yamane, Yoshinobu;Tsukamoto, Shuntaro;Azuma, Hiroshi;Nagao, Satoshi;Murai, Norio;Nishibata, Kyoko;Fukushima, Sayo;Ichikawa, Kenji;Nakagawa, Takayuki;Hata Sugi, Naoko;Ito, Daisuke;Kato, Yu;Goto, Aya;Kakiuchi, Dai;Ueno, Takashi;Matsui, Junji;Matsushima, Tomohiro [Bioorganic and Medicinal Chemistry,2021,vol. 39,art. no. 116137]
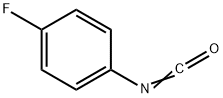
1195-45-5
204 suppliers
$6.00/1g

1437323-26-6
22 suppliers
inquiry
![2-[3-(4-fluorophenyl)ureidomethylene]malonic acid diethyl ester](/CAS/20180703/GIF/1437323-24-4.gif)
1437323-24-4
5 suppliers
inquiry

1437323-26-6
22 suppliers
inquiry
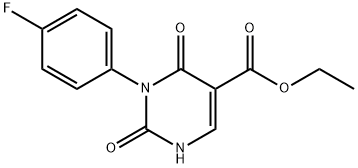
1437323-25-5
9 suppliers
inquiry

1437323-26-6
22 suppliers
inquiry
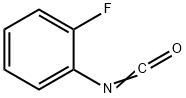
16744-98-2
134 suppliers
$15.00/1g

1437323-26-6
22 suppliers
inquiry