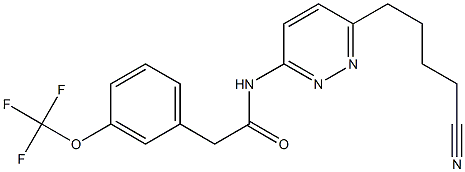
N-[6-(4-cyanobutyl)pyridazin-3-yl]-2-[3-(trifluoromethoxy)phenyl]acetamide synthesis
- Product Name:N-[6-(4-cyanobutyl)pyridazin-3-yl]-2-[3-(trifluoromethoxy)phenyl]acetamide
- CAS Number:1439400-48-2
- Molecular formula:C18H17F3N4O2
- Molecular Weight:378.3484

226570-68-9
22 suppliers
$276.14/50ml

1439400-46-0
15 suppliers
inquiry
![N-[6-(4-cyanobutyl)pyridazin-3-yl]-2-[3-(trifluoromethoxy)phenyl]acetamide](/CAS/20180601/GIF/1439400-48-2.gif)
1439400-48-2
2 suppliers
inquiry
Yield:1439400-48-2 92%
Reaction Conditions:
with 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) in tetrahydrofuran at 20; for 4 h;Inert atmosphere;
Steps:
1
4-Cyanobutylzinc bromide solution (3.0 equiv., 0.50 mol, 1.0 L) was charged into an argon gas purged 5000 mL 3 neck round bottom flask. Argon(g) purge for 5 minutes followed by the addition of 1117 (1.0 equiv., 0.167 mol, 55.3 g) and NiCl2(dppp) (0.15 equiv., 0.0251 mol, 13.6 g) under a blanket of argon(g). The reaction usually goes to completion after 4 hours (TLC: 1 : 1 hexanes-ethyl acetate). EtOAc (15 vol., 832 mL) added to deep red solution. Water (15 vol., 832 mL) was added, thick slurry formed. IN HC1 added until slurry breaks to pale blue layer (~6 vol., 333 mL). Transferred to separatory funnel and organic layer was washed with IN HC1 (2x500 mL), dried (MgSC^) and concentrated by rotary evaporation (bath < 30 °C) to a solid reddish oil. Oil dissolved in dichloromethane (15 vol., 832 mL), silica gel (100g) was slurried into red solution, this was concentrated by rotary evaporation (bath < 30 °C) to a solid reddish powder. Loaded onto a bed of silica gel (5 cm x 11 cm), flushed with 25% hexanes in ethyl acetate (3 L), combined organics concentrated by rotary evaporation (bath < 30 °C). Dried under high vacuum to a constant weight to afford N-(6-(4-cyanobutyl)pyridazin-3 -yl)-2-(3 -(trifluoromethoxy)phenyl)acetamide 1118: yield of 58.2 g (92%). 1H NMR (300 MHz, DMSO-d6) δ 11.41 (s, 1H), 8.28(d, J=9.2 Hz, 1H), 7.65(d, J=9.2 Hz, 1H), 7.52 - 7.27(m, 4H), 3.89(s, 2H), 2.92(t, J=7.5 Hz, 2H), 2.56(t, J=7.0 Hz, 2H), 1.80 (m, 2H), 1.61 (m, 2H).
References:
WO2013/78123,2013,A1 Location in patent:Page/Page column 147

203302-97-0
167 suppliers
$6.00/250mg
![N-[6-(4-cyanobutyl)pyridazin-3-yl]-2-[3-(trifluoromethoxy)phenyl]acetamide](/CAS/20180601/GIF/1439400-48-2.gif)
1439400-48-2
2 suppliers
inquiry

1439400-46-0
15 suppliers
inquiry
![N-[6-(4-cyanobutyl)pyridazin-3-yl]-2-[3-(trifluoromethoxy)phenyl]acetamide](/CAS/20180601/GIF/1439400-48-2.gif)
1439400-48-2
2 suppliers
inquiry