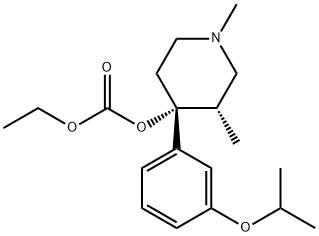
Carbonic acid, (3S,4R)-1,3-diMethyl-4-[3-(1-Methylethoxy)phenyl]-4-piperidinyl ethyl ester (9CI) synthesis
- Product Name:Carbonic acid, (3S,4R)-1,3-diMethyl-4-[3-(1-Methylethoxy)phenyl]-4-piperidinyl ethyl ester (9CI)
- CAS Number:143957-08-8
- Molecular formula:C19H29NO4
- Molecular Weight:335.44
Yield:-
Reaction Conditions:
with sodium hydroxide in ethyl acetate;
Steps:
2 EXAMPLE 2
EXAMPLE 2 Preparation of cis-(+-)carbonic acid ethyl 1,3-dimethyl-4-[3-(l-methylethoxy)phenyl]-4-piperidinyl ester. (Formula II where R1, R2 and R5 are methyl, R4 is hydrogen and Ar is 3-(1-methylethoxy)phenyl. 4-Hydroxypiperidine (463 g, 1.758 mol) from Example 1 was combined with ethyl acetate (2275 ml) under nitrogen. The solution was cooled to 0°-5° C. and ethyl chloroformate (205 ml, 2.144 mol) was added while maintaining the temperature below 15° C. The reaction mixture was stirred for an additional 3 hours at room temperature. The mixture was then added to 5N NaOH (750 ml) with stirring (to pH of 12-13). The organic layer was separated and washed with de-ionized water. Solvent was removed by evaporation at 50° C. to provide 591 g of the named product is a viscous oil. Analysis: Calc. for (C19 H29 NO4): C, 68.03; H, 8.71; N, 4.18. Found: C, 67.82; H, 8.86; N, 4.35.
References:
US5136040,1992,A
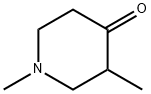
4629-80-5
212 suppliers
$7.00/5g
![Carbonic acid, (3S,4R)-1,3-diMethyl-4-[3-(1-Methylethoxy)phenyl]-4-piperidinyl ethyl ester (9CI)](/CAS/20150408/GIF/143957-08-8.gif)
143957-08-8
3 suppliers
inquiry
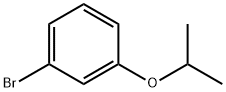
131738-73-3
146 suppliers
$30.00/1g
![Carbonic acid, (3S,4R)-1,3-diMethyl-4-[3-(1-Methylethoxy)phenyl]-4-piperidinyl ethyl ester (9CI)](/CAS/20150408/GIF/143957-08-8.gif)
143957-08-8
3 suppliers
inquiry
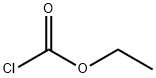
![4-Piperidinol, 1,3-diMethyl-4-[3-(1-Methylethoxy)phenyl]-](/CAS/20150408/GIF/145340-44-9.gif)