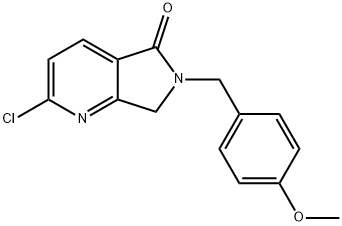
2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE synthesis
- Product Name:2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE
- CAS Number:1440519-73-2
- Molecular formula:C15H13ClN2O2
- Molecular Weight:288.73

1093879-99-2
9 suppliers
$60.00/5mg
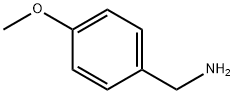
2393-23-9
463 suppliers
$20.00/25g
![2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE](/CAS/20180703/GIF/1440519-73-2.gif)
1440519-73-2
26 suppliers
$65.00/10mg
Yield:1440519-73-2 55%
Reaction Conditions:
in tetrahydrofuran at 20; for 4 h;
Steps:
A.5 Step 5:
To a solution of (4-methoxyphenyl)methanamine (2.49 g, 18.15 mmol) in tetrahydrofuran (50 mL ) was added methyl 6-chloro-2-(chloromethyl)pyridine-3- carboxylate (4.0 g, 18.17 mmol). The mixture was stirred at room temperature for 4 h. The mixture was concentrated under vacuum. The residue was purified by flash column chromatography with 0-25% ethyl acetate in petroleum ether to afford 2-chloro-6-[(4- methoxyphenyl)methyl]-7H-pyrrolo[3,4-b]pyridin-5-one (2.90 g, 55%) as a yellow solid. MS m/z 289.0 [M+1]+.
References:
WO2022/20342,2022,A1 Location in patent:Page/Page column 40; 57
65719-09-7
191 suppliers
$21.00/1g
![2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE](/CAS/20180703/GIF/1440519-73-2.gif)
1440519-73-2
26 suppliers
$65.00/10mg
1033809-42-5
1 suppliers
inquiry
![2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE](/CAS/20180703/GIF/1440519-73-2.gif)
1440519-73-2
26 suppliers
$65.00/10mg
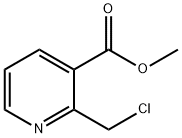
177785-14-7
46 suppliers
$185.00/100mg
![2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE](/CAS/20180703/GIF/1440519-73-2.gif)
1440519-73-2
26 suppliers
$65.00/10mg
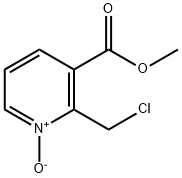
1093879-98-1
1 suppliers
inquiry
![2-CHLORO-6-(4-METHOXYBENZYL)-6,7-DIHYDROPYRROLO[3,4-B]PYRIDIN-5-ONE](/CAS/20180703/GIF/1440519-73-2.gif)
1440519-73-2
26 suppliers
$65.00/10mg