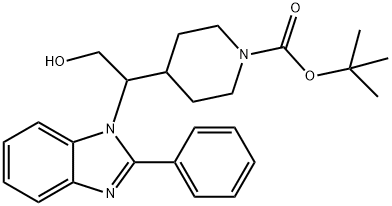
1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester
- CAS Number:1440753-74-1
- Molecular formula:C25H31N3O3
- Molecular Weight:421.53
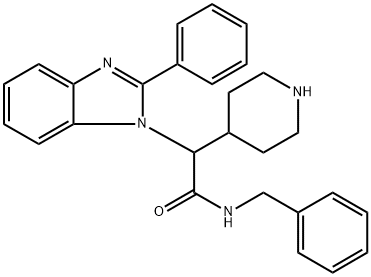
aMino]-2-oxo-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-63-8.gif)
1440753-63-8
7 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-74-1.gif)
1440753-74-1
10 suppliers
inquiry
Yield:1440753-74-1 80%
Reaction Conditions:
with sodium tetrahydroborate;ethanol in tetrahydrofuran at 20; for 16 h;
Steps:
17 Example 17
Example 17
Preparation of tert-Butyl 4-(2-hydroxy-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)ethyl)piperidine-1-carboxylate (S17)
A solution of Boc-protected amide S15 (1.0 g, 1.6 mmol) in EtOH:THF (1:5, 15 mL) was added to a suspension of NaBH4 (242 mg, 6.4 mmol) in THF (15 mL) at room temperature over 1 h.
The reaction mixture was stirred at room temperature for 12 h. NaBH4 (120 mg, 3.2 mmol) was added to the reaction mixture and stirring was continued for 3 h.
To the reaction mixture was added NH4Cl-solution (40 mL), followed by CH2Cl2 (30 mL).
The pH of the aqueous layer was adjusted to 8 by addition of sat. NaHCO3-solution.
The aqueous layers were reextracted with CH2Cl2 (3x 30 mL).
The combined organic layers were concentrated in vacuo and the residue was purified by flash chromatography (gradient cyclohexane:
ethyl acetate 10:1 to 1:2) to afford the product (538 mg, 1.27 mmol, 80%).
1H NMR (400 MHz, DMSO-d6) δ 7.87 - 7.78 (m, 1H), 7.74 (d, J = 3.6 Hz, 2H), 7.70 - 7.62 (m, 1H), 7.58 - 7.46 (m, 3H), 7.26 - 7.11 (m, 2H), 5.29 - 5.07 (m, 1H), 4.30 - 4.07 (m, 2H), 4.04 - 3.73 (m, 2H), 3.71 - 3.44 (m, 1H), 2.79 - 2.56 (m, 1H), 2.45 - 2.23 (m, 2H), 1.81 (d, J = 12.2 Hz, 1H), 1.46 - 1.20 (m, 10H), 0.93 (d, J = 10.4 Hz, 1H), 0.68 - 0.38 (m, 2H).
13C NMR (101 MHz, DMSO-d6) δ 155.95, 154.28, 143.86, 134.19, 131.67, 130.82, 130.09, 129.08, 122.74, 122.40, 120.11, 113.62, 79.18, 64.71, 60.33, 35.71, 30.18, 29.06, 28.67.LC-MS (ESI): calcd for C25H31N3O3: 422.24382 [M+H]+, found 422.20 [M+H]+, Rt = 6.31 min; HR-MS found 422.24271.
References:
EP2698367,2014,A1 Location in patent:Paragraph 0099-0100
1440753-53-6
10 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-74-1.gif)
1440753-74-1
10 suppliers
inquiry

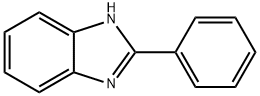
24424-99-5
822 suppliers
$13.50/25G
![1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-74-1.gif)
1440753-74-1
10 suppliers
inquiry
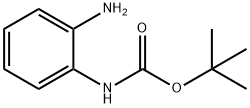
716-79-0
302 suppliers
$6.00/5g
![1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-74-1.gif)
1440753-74-1
10 suppliers
inquiry
146651-75-4
133 suppliers
$6.00/250mg
![1-Piperidinecarboxylic acid, 4-[2-hydroxy-1-(2-phenyl-1H-benziMidazol-1-yl)ethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/1440753-74-1.gif)
1440753-74-1
10 suppliers
inquiry