
[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]azanium:2-hydroxy-2-phenylacetate synthesis
- Product Name:[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]azanium:2-hydroxy-2-phenylacetate
- CAS Number:1444301-72-7
- Molecular formula:C17H17F2NO3
- Molecular Weight:321.32
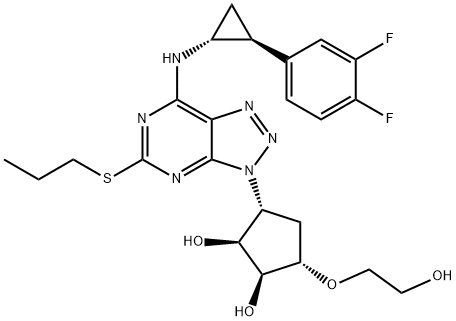
![2-[[(3aR,4S,6R,6aS)-6-(7-azanyl-5-propylsulfanyl-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,2-dimethyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]ethanol](/CAS/20180629/GIF/1354945-69-9.gif)
1354945-69-9
28 suppliers
inquiry
![[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]azanium:2-hydroxy-2-phenylacetate](/CAS/20180527/GIF/1444301-72-7.gif)
1444301-72-7
2 suppliers
inquiry
274693-27-5
626 suppliers
$13.00/10mg
Yield:274693-27-5 93%
Reaction Conditions:
with sodium carbonate in acetonitrile at 20; for 20 h;
Steps:
45 Example 45: Preparation of ticagrelor (TCG)
To a stirring suspension of (1 fl,2S)-2-(3,4-difluorophenyl)cyclopropanamonium mandelate (0.33 g, 1 .03 mmol) and sodium carbonate (0.27 g, 2.5 mmol) in acetonitrile (12 mL) at 20 °C was added (1 S,2S,3fl,5S)-3-(7-(((1 ft,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5- (propylthio)-3/-/-[1 ,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1 ,2-diol (CLTOL; 0.39 g, 1 mmol). The mixture was stirred for 20 h, diluted with water (50 mL) and extracted with ethyl acetate (60 mL). The extract was washed with water (50 mL), 0.1 M aqueous acetic acid (50 mL), again water (50 mL) and evaporated under reduced pressure to give ticagrelor (0.50 g, 93% yield): 19F NMR (CDCI3, 470.5 MHz) δ -142.5 (m, 1 F), -139.0 (m, 1 F); MS (ESI) m/z: 523 [MH]+.
References:
WO2013/92900,2013,A1 Location in patent:Page/Page column 65; 66
![[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]azanium:2-hydroxy-2-phenylacetate](/CAS/20180527/GIF/1444301-72-7.gif)
1444301-72-7
2 suppliers
inquiry
1265911-54-3
1 suppliers
inquiry