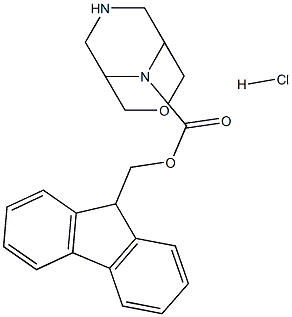
(9H-fluoren-9-yl)methyl 3-oxa-7,9-diazabicyclo[3.3.1]nonane-9-carboxylate hydrochloride synthesis
- Product Name:(9H-fluoren-9-yl)methyl 3-oxa-7,9-diazabicyclo[3.3.1]nonane-9-carboxylate hydrochloride
- CAS Number:1445804-25-0
- Molecular formula:C21H23ClN2O3
- Molecular Weight:386.8719
![3-Oxa-7,9-diazabicyclo[3.3.1]nonane-7,9-dicarboxylic acid, 7-(1,1-dimethylethyl) 9-(9H-fluoren-9-ylmethyl) ester](/CAS/20210305/GIF/1445804-24-9.gif)
1445804-24-9
0 suppliers
inquiry
![(9H-fluoren-9-yl)methyl 3-oxa-7,9-diazabicyclo[3.3.1]nonane-9-carboxylate hydrochloride](/CAS/20180531/GIF/1445804-25-0.gif)
1445804-25-0
9 suppliers
inquiry
Yield:1445804-25-0 92%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 1.5 h;
Steps:
Step 2 : 3-oxa-7.9-diaza-bicvclor3.3.11nonane-9-carboxylic acid 9H-fluoren-9-ylmethyl ester hydrochloride
3-Oxa-7,9-diaza-bicyclo[3.3.1]nonane-7,9-dicarboxylic acid 7-tert-butyl ester 9-(9H- fluoren-9-ylmethyl) ester (1.29 g, 2.76 mmol) was dissolved into a 4N solution of HCI in 1 ,4-dioxane (10 mL). The resulting mixture was stirred at RT for 1.5 hours, and then concentrated under vacuum to give a colorless oil. The oil was taken up with Et20 (20 ml) and a precipitation occurred. The precipitate was filtered off, washed with Et20 (2x) and dried under vacuum to give the title compound as a white powder (1.06 g, 92%). 1H NMR (300 MHz, DMSO-d6) δ 9.81 (s, 1 H), 8.22 (s, 1H), 7.90 (d, J = 7.3 Hz, 2H), 7.64 (d, J = 7.4 Hz, 2H), 7.47-7.31 (m, 4H), 4.61-4.44 (m, 2H), 4.31 (t, J = 6.0 Hz, 1 H), 4.07 (s, 1 H), 4.02-3.84 (m, 3H), 3.62-3.53 (m, 1 H), 3.51-3.37 (m, 3H), 3.09-2.90 (m, 2H). HPLC (max plot) 97.4%; Rt 2.94 min. UPLC/MS: (MS+) 351.3 ([M+H]+).
References:
WO2013/91773,2013,A1 Location in patent:Page/Page column 83
![7-Boc-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-41-9.gif)
864448-41-9
48 suppliers
$175.00/1mg
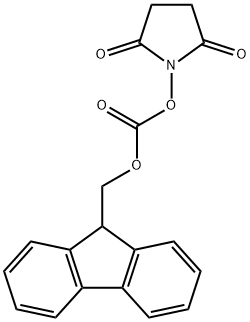
82911-69-1
606 suppliers
$7.00/25g
![(9H-fluoren-9-yl)methyl 3-oxa-7,9-diazabicyclo[3.3.1]nonane-9-carboxylate hydrochloride](/CAS/20180531/GIF/1445804-25-0.gif)
1445804-25-0
9 suppliers
inquiry