
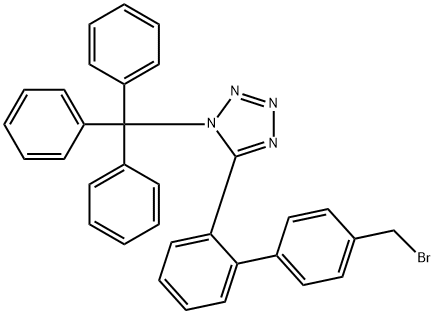
diethyl 2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-4,5-dicarboxylate synthesis
- Product Name:diethyl 2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-4,5-dicarboxylate
- CAS Number:144690-53-9
- Molecular formula:C45H42N6O4
- Molecular Weight:730.85
124750-51-2
461 suppliers
$6.00/1g
144689-94-1
276 suppliers
$8.00/5g
![diethyl 2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-4,5-dicarboxylate](/CAS/20180629/GIF/144690-53-9.gif)
144690-53-9
43 suppliers
inquiry
Yield:-
Reaction Conditions:
in N,N-dimethyl acetamide;
Steps:
35.a 35(a)
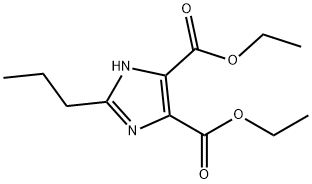
35(a) Diethyl 2-propyl-1-{4-[2-(trityltetrazol-5-yl)phenyl]phenyl}methylimidazole-4,5-dicarboxylate 0.441 g of potassium t-butoxide was added to a solution of 1.00 g of diethyl 2-propylimidazole-4,5-dicarboxylate (prepared as described in Preparation 12) in 15 ml of N,N-dimethylacetamide, and the resulting mixture was stirred at room temperature for 30 minutes. A solution of 2.19 g of 4-[2-(trityltetrazol-5-yl)phenyl]benzyl bromide in 15 ml of N,N-dimethylacetamide was then added dropwise to the reaction mixture at room temperature, and the reaction mixture was stirred at room temperature for 3 hours. At the end of this time, it was diluted with water and then extracting with ethyl acetate. The extract was dried over anhydrous magnesium sulfate and then freed from the solvent by distillation. The residue was purified by column chromatography through silica gel, using a 1:1 by volume mixture of hexane and ethyl acetate as the eluent, to give 2.24 g of the title compound as an amorphous solid. Nuclear Magnetic Resonance Spectrum (CDCl3) δ ppm: 0.88 (3H, triplet, J=7.5 Hz); 1.20 (3H, triplet, J=7.5 Hz); 1.39 (3H, triplet, J=7.5 Hz); 1.59 (6H, singlet); 1.61-1.72 (2H, multiplet); 2.55 (2H, triplet, J=7.5 Hz); 4.20 (2H, quartet, J=7.5 Hz); 4.39 (2H, quartet, J=7.5 Hz); 5.30 (2H, singlet); 6.78 (2H, doublet, J=8 Hz); 6.92-7.52 (20H, multiplet); 7.90 (1H, doublet, J=7.5 Hz).
References:
US5616599,1997,A