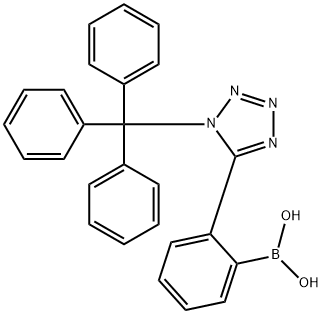
[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID synthesis
- Product Name:[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID
- CAS Number:144873-97-2
- Molecular formula:C26H21BN4O2
- Molecular Weight:432.28
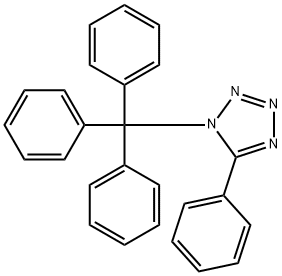
154750-11-5
14 suppliers
$503.27/5MG

5419-55-6
370 suppliers
$12.00/5g
![[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID](/CAS/GIF/144873-97-2.gif)
144873-97-2
16 suppliers
inquiry
Yield:144873-97-2 94%
Reaction Conditions:
Stage #1: N-(triphenylmethyl)phenyltetrazolewith n-butyllithium in tetrahydrofuran;hexane at -25 - -5; for 3.75 h;
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at -25 - 35; for 2 h;
Stage #3: with water;acetic acid in tetrahydrofuran;hexane at 0 - 5; for 1 - 1.33333 h;Product distribution / selectivity;
Steps:
7
Example 7: Preparation of 2-[5-(l-trityl-lH-tetra2ol') phenylboronic acid.; [0048] A solution of 5-phenyl-l-trityl-lH-tetrazole (100.0 g, 0.26 mol) in dry tetrahydrofuran ("THF") (800.0 ml) was cooled to about -25°C under nitrogen or argon, n- Butyl lithium (1.6M in hexane) was added to quench the traces of water in the reaction mixture until the color of the reaction mixture remained red for at least 5 minutes. n-Butyl lithium (180.0 ml) was then added dropwise to the reaction mixture over a period of about 45-50 minutes at a temperature below -25°C. The temperature of the reaction mixture was raised to -5°C. The reaction mixture was stirred at -5°C for 3 hrs. The reaction mixture was cooled to -25°C and triisopropyl borate (101.0 ml) was added. The reaction mixture was warmed to a temperature of about 25-35°C and the reaction mixture was stirred for 2 hrs. The reaction mixture was cooled again to 0-5°C and 3% aq. acetic acid (667.0 ml) was slowly added over 30-40 minutes. The reaction mixture was stirred for 30-40 minutes and then filtered. The product was washed with 500 ml water. The wet material was dried at about 45°C under reduced pressure of about 500-760 mm Hg until constant weight. [Yield: 94%; Purity: 94% area by HPLC]
References:
WO2008/27385,2008,A2 Location in patent:Page/Page column 14

154750-11-5
14 suppliers
$503.27/5MG
![[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID](/CAS/GIF/144873-97-2.gif)
144873-97-2
16 suppliers
inquiry
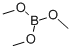
121-43-7
346 suppliers
$14.00/25mL
![[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID](/CAS/GIF/144873-97-2.gif)
144873-97-2
16 suppliers
inquiry

154750-11-5
14 suppliers
$503.27/5MG
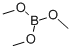
121-43-7
346 suppliers
$14.00/25mL
![[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID](/CAS/GIF/144873-97-2.gif)
144873-97-2
16 suppliers
inquiry
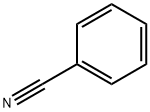
100-47-0
359 suppliers
$14.14/25gm:
![[2-[1-(TRIPHENYLMETHYL)-1H-TETRAZOL-5-YL]PHENYL]BORONIC ACID](/CAS/GIF/144873-97-2.gif)
144873-97-2
16 suppliers
inquiry