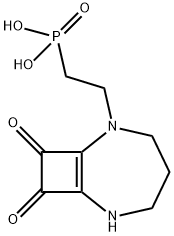
2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid synthesis
- Product Name:2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid
- CAS Number:144912-63-0
- Molecular formula:C9H13N2O5P
- Molecular Weight:260.18
![Phosphonic acid, P-[2-((8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl))ethyl]-, diethyl ester](/CAS/20210305/GIF/144912-83-4.gif)
144912-83-4
2 suppliers
inquiry
![2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid](/CAS/20150408/GIF/144912-63-0.gif)
144912-63-0
23 suppliers
$202.00/10mg
Yield:144912-63-0 87%
Reaction Conditions:
in dichloromethane;water;acetone;
Steps:
5 [2-(8,9-Dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethyl]-phosphonic Acid
EXAMPLE 5 [2-(8,9-Dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethyl]-phosphonic Acid Under N2 bromotrimethylsilane (83 mL, 96.3 g, 0.63 mole; Aldrich 19,440-9) was added dropwise at a fast rate to a solution of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethyl]phosphonic acid diethyl ester (37.6 g, 0.12 mole) in methylene chloride (350 mL). The reaction mixture was kept in a water bath at approximately 20° C. for 15 hr. The clear solution was concentrated in vacuo and the foamy residue was taken up in acetone (600 mL) with vigorous shaking to result in a thin suspension. Water (50 mL, 2.78 moles) was added to give a gummy precipitate which solidified instantly. The suspension was shaken vigorously for 10 minutes, filtered and washed with acetone to give a yellow solid compound. The solids were taken up in boiling water (450 mL) and the hot solution was filtered through a fluted filter paper to remove a small amount of insoluble material. The clear aqueous solution was cooled in ice and crystallization began at once. The thick crystalline mass was diluted by slow addition of acetone (800 mL), kept cold for 1 hr, filtered and washed with acetone and then hexane to give the title compound as a pale yellow solid (20.2 g). A second crop from the mother liquor (100% purity by LC) yielded an additional amount (6.5 g) for a total yield of 87%. NMR (DMSO-d6, 400 Mhz): 1.90 (m, 4H), 3.25 (m, 2H), 3.36 (m, 2H), 3.84 (q, 2H), 8.45 (s, 1H). LC analysis: (Column: Nova Pak C18, 300*3.9 mm; Eluent: 20/80 MeOH/0.005 M Pic A; Flowrate: 1 mL/min; UV detectors at 210 nm). Analysis: Calc'd. for C9 H13 N2 O5 P.0.1 H2 O: C, 41.26; H, 5.08; N, 10.69%; Found: C, 41.17; H, 5.04; N, 10.42%; Karl-Fischer analysis: 0.55% H2 O; MS: -FAB [M-H]- m/z 259.
References:
US5990307,1999,A

144912-81-2
1 suppliers
inquiry
![2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid](/CAS/20150408/GIF/144912-63-0.gif)
144912-63-0
23 suppliers
$202.00/10mg

144912-82-3
1 suppliers
inquiry
![2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid](/CAS/20150408/GIF/144912-63-0.gif)
144912-63-0
23 suppliers
$202.00/10mg

200441-27-6
1 suppliers
inquiry
![2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid](/CAS/20150408/GIF/144912-63-0.gif)
144912-63-0
23 suppliers
$202.00/10mg

200441-28-7
1 suppliers
inquiry
![2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)ethylphosphonic acid](/CAS/20150408/GIF/144912-63-0.gif)
144912-63-0
23 suppliers
$202.00/10mg