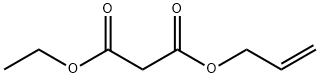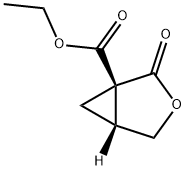
ethyl (1S,5R)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate synthesis
- Product Name:ethyl (1S,5R)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate
- CAS Number:145032-58-2
- Molecular formula:C8H10O4
- Molecular Weight:170.16
Yield:-
Steps:
Multi-step reaction with 2 steps
1: dichloromethane / 2 h / 20 °C / Inert atmosphere; Schlenk technique
2: silver(I) triflimide; (3aR,3a''R,8aR,8a''R)-N6,N6,N6'',N6''-tetrakis((R)-1-phenylethyl)-4,4,4'',4'',8,8,8'',8''-octa-4-(tert-butyl)phenyl octahydrodispiro[[1,3]dioxolo[4,5-e][1,3,2]dioxaphosphepine-2,1'-cyclohexane-4',2''-[1,3]dioxolo[4,5-e][1,3,2]dioxaphosphepine]-6,6''-diamine bisgoldchloride / toluene / 1 h / 75 °C / Inert atmosphere; Schlenk technique
References:
Klimczyk, Sebastian;Misale, Antonio;Huang, Xueliang;Maulide, Nuno [Angewandte Chemie - International Edition,2015,vol. 54,# 35,p. 10365 - 10369][Angew. Chem.,2015,vol. 127,# 35,p. 10507 - 10511,5]
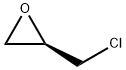
51594-55-9
357 suppliers
$10.00/1g
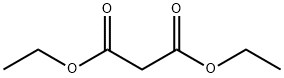
105-53-3
736 suppliers
$5.00/25g
![ethyl (1S,5R)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate](/CAS/20180601/GIF/145032-58-2.gif)
145032-58-2
23 suppliers
inquiry
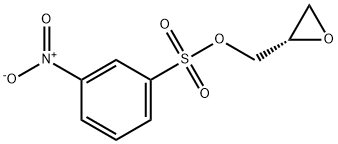
115314-14-2
254 suppliers
$5.00/250mg

105-53-3
736 suppliers
$5.00/25g
![ethyl (1R,5S)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate](/CAS/20180527/GIF/184838-77-5.gif)
184838-77-5
26 suppliers
inquiry
![ethyl (1S,5R)-2-oxo-3-oxabicyclo[3.1.0]hexane-1-carboxylate](/CAS/20180601/GIF/145032-58-2.gif)
145032-58-2
23 suppliers
inquiry