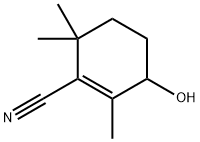
3-Hydroxy-2,6,6-triMethyl-1-cyclohexene-1-carbonitrile synthesis
- Product Name:3-Hydroxy-2,6,6-triMethyl-1-cyclohexene-1-carbonitrile
- CAS Number:145106-79-2
- Molecular formula:C10H15NO
- Molecular Weight:165.23
![1,3,3-TriMethyl-7-oxabicyclo[4.1.0]heptane-2-carbonitrile](/CAS/GIF/264279-20-1.gif)
264279-20-1
7 suppliers
inquiry

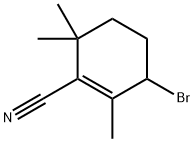
145106-79-2
8 suppliers
inquiry
Yield:145106-79-2 77.3%
Reaction Conditions:
with sodium in methanol at 0 - 20;
Steps:
Preparation of Compound-6:
To a stirred solution of sodium (1.25 g, 54 mmol) in methanol(10 mL) at 0 °C was slowly added methanol (450 mL) followed by Compound-5 (30 g, 180mmol) in methanol (10 mL). The resulting solution was stirred at room temperature overnight,and then evaporated under vacuum. The residue was diluted with water, neutralized with acetic acid and extracted thrice with ethyl acetate. The combined organic layers were washed with brine, dried over anhydrous Na2SO4 and concentrated. The crude compound was purified by column chromatography on 60-120 mesh silica gel and eluted with 20% ethyl acetate/petroleumether to obtain Compound-6 (23 g, 77.3%). MS (m/z): [M + H]+, 166.2.
References:
Zhang, Jianye;Dong, Zhiqian;Mundla, Sreenivasa Reddy;Hu, X. Eric;Seibel, William;Papoian, Ruben;Palczewski, Krzysztof;Golczak, Marcin [Molecular Pharmacology,2015,vol. 87,# 3,p. 477 - 491] Location in patent:supporting information
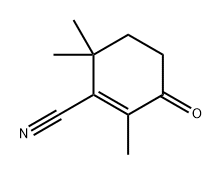
56830-39-8
0 suppliers
inquiry

145106-79-2
8 suppliers
inquiry
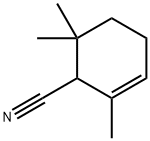
57524-13-7
9 suppliers
inquiry

145106-79-2
8 suppliers
inquiry
110-93-0
299 suppliers
$8.00/25g

145106-79-2
8 suppliers
inquiry