
tert-Butyl 4-(4-hydroxypyridin-2-yl)piperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-hydroxypyridin-2-yl)piperazine-1-carboxylate
- CAS Number:1453265-70-7
- Molecular formula:C14H21N3O3
- Molecular Weight:279.33
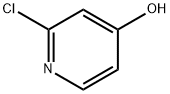
17368-12-6
276 suppliers
$4.00/1g

57260-71-6
735 suppliers
$5.00/5g

1453265-70-7
8 suppliers
inquiry
Yield:1453265-70-7 44%
Reaction Conditions:
in butan-1-ol at 140; for 72 h;
Steps:
3.A
Step A: To a solution of 2-chloro-4-hydroxypyridine (12.9 g, 100 mmol) in n-BuOH (50 mL) was added t-butyl piperazine-l-carboxylate (46.5 g, 250 mmol). The mixture was heated at 140 °C for 3 days and then diluted with EtOAc (2 L) at ambient temperature. The organic phase was washed with saturated NH4C1 solution and then brine. The organic layer was separated, dried over MgSC^, and concentrated under reduced pressure. The residue was purified by column chromatography (5%-20% MeOH/CH2Cl2) to give 12.2 g (44%) of i-butyl 4- (4-hydroxypyridin-2-yl)piperazine-l-carboxylate as a white solid. MS m/z 280.2 [M+H]+; 1H NMR (500 MHz, CDC13) δ (ppm) 7.72 (1H, d, J= 6.62 Hz), 6.20 (1H, dd, J= 6.31, 2.21 Hz), 6.02 (1H, d, J= 1.89 Hz), 3.56 (4H, m), 3.42 (4H, m), 1.50 (9H, s).
References:
WO2013/130689,2013,A1 Location in patent:Paragraph 00917; 00918